Metformin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- METFORMIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METFORMIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- METFORMIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL PATIENT PACKAGE INSERT
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
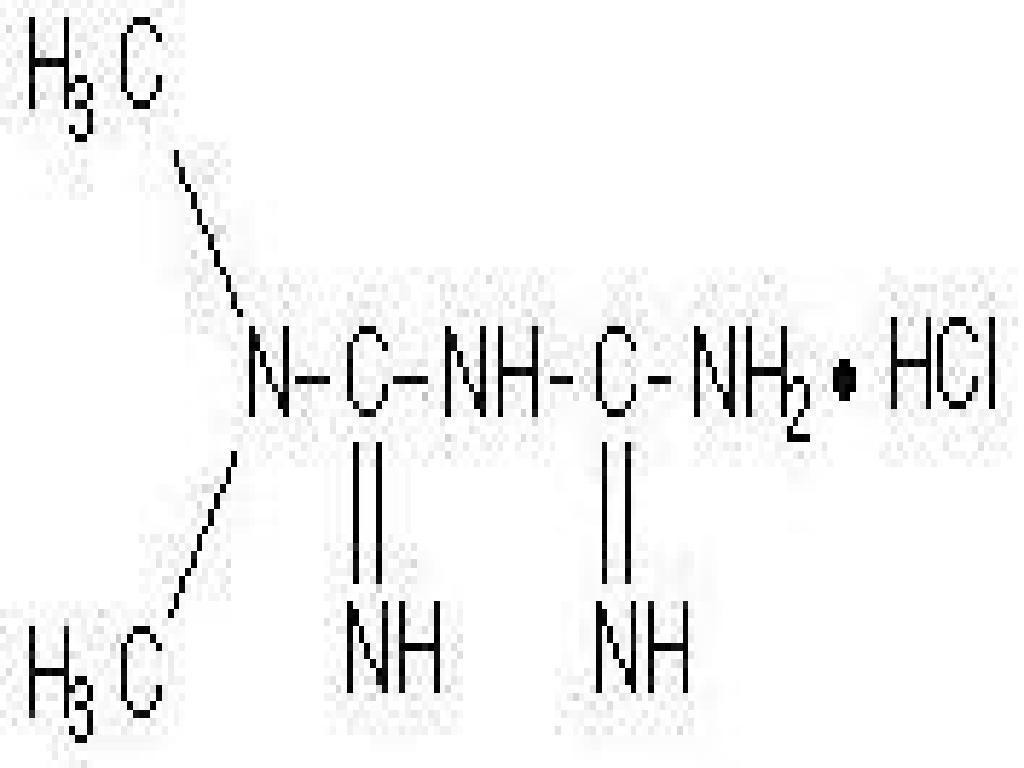
METFORMIN HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPRECAUTIONS
Pharmacokinetics
Absorption and Bioavailability
Distribution
Metabolism and Elimination
Table 1
Special Populations
Patients with Type 2 Diabetes
Table 1
Renal Insufficiency
Table 1WARNINGS
Hepatic Insufficiency
Geriatrics
WARNINGSDOSAGE AND ADMINISTRATION
Subject Groups: MetforminCmaxbTmaxcRenalHydrochloride Tablets(mcg/mL)(hrs)Clearancedosea (number of subjects)(mL/min)Healthy, nondiabetic adults:+++++++++Adults with type 2 diabetes:++++++Elderlyf, healthy nondiabetic adults:Renal-impaired adults:850 mg single dose+++++++++
Pediatrics
Gender
Race
Clinical Studies
MetforminHydrochloridePlacebop-ValueTablets(n=145)(n=141)FPG (mg/dL)Hemoglobin A1c(%)Body Weight (lbs)
CombGlybMETp-values(n=213)(n=209)(n=201)Glyb vsMET vsMETCombCombGlybFasting PlasmaGlucose (mg/dL)Hemoglobin A1c (%)Body Weight (lbs)
Metformin HClCombined Metformin HydrochlorideTablets vs PlaceboTablets/Glyburide vs MonotherapyMetforminMetforminMetforminHClPlaceboHClHClGlyburideTablets(n=145)TabletsTablets/(n=209)(n-141)(n=210)Glyburide(n=213)Total Cholesterol (mg/dL)Total Triglycerides (mg/dL)LDL-Cholesterol (mg/dL)HDL Cholesterol (mg/dL)
Tables 23
MetforminHydrochloridePlacebo/TreatmentTablets/ InsulinInsulinDifference(n=26)(n=28)Mean+SEHemoglobin A1c (%)Baseline8.959.32Change at FINAL VISIT-2.1-1.56-0.54+0.43aInsulin Dose (U/day)Baseline93.1294.64Change at FINAL VISIT-0.1515.93-16.08+7.77ba Statistically significant using analysis of covariance with baseline as covariate (p=0.04)
Not significant using analysis of variance (values shown in table)
b Statistically significant for insulin (p=0.04)
A second double-blind, placebo-controlled study (n=51), with 16 weeks of randomized treatment, demonstrated that in patients with type 2 diabetes controlled on insulin for 8 weeks with an average HbA1c of 7.460.97%, the addition of metformin hydrochloride tablets maintained similar glycemic control (HbA1c 7.150.61 versus 6.970.62 for metformin hydrochloride tablets plus insulin and placebo plus insulin, respectively) with 19% less insulin versus baseline (reduction of 23.6830.22 versus an increase of 0.4325.2 units for metformin hydrochloride tablets plus insulin and placebo plus insulin, p<0.01). In addition, this study demonstrated that the combination of metformin hydrochloride tablets plus insulin resulted in reduction in body weight of 3.114.3 lbs, compared to an increase of 1.36.08 lbs for placebo plus insulin, p=0.01.
Pediatric Clinical Studies
Metformin HydrochloridePlacebop-ValueTabletsFPG (mg/dL)(n=37)(n=36)Body Weight (lbs)(n=39)(n=38)
INDICATIONS & USAGE
METFORMIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGSPRECAUTIONS
PRECAUTIONS
WARNINGS
Lactic AcidosisPRECAUTIONS
PRECAUTIONS
PRECAUTIONS
CONTRAINDICATIONSPRECAUTIONS
PRECAUTIONS
GeneralWARNINGSDOSAGE AND ADMINISTRATION
PRECAUTIONS: Drug Interactions
CONTRAINDICATIONS
PRECAUTIONS: Laboratory Tests
WARNINGS
LABORATORY TESTS
DOSAGE AND ADMINISTRATIONDRUG INTERACTIONS
DOSAGE AND ADMINISTRATION: Concomitant Metformin and Oral Sulfonylurea Therapy in Adult PatientsCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic effectsNURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGY: Pediatric Clinical StudiesADVERSE REACTIONS: Pediatric PatientsDOSAGE AND ADMINISTRATION: Recommended Dosing Schedule: Pediatrics
GERIATRIC USE
CONTRAINDICATIONSWARNINGSCLINICAL PHARMACOLOGY: PharmacokineticsWARNINGSDOSAGE AND ADMINISTRATIONMETFORMIN HYDROCHLORIDE ADVERSE REACTIONS
Adverse ReactionMetformin HydrochlorideTabletsPlaceboMonotherapy(n=145)(n=141)% Patients
Pediatric Patients
OVERDOSAGE
WARNINGSDOSAGE & ADMINISTRATION
Recommended Dosing Schedule
Recommended Dosing Schedule
Transfer From Other Antidiabetic Therapy
Concomitant Metformin and Oral Sulfonylurea Therapy in Adult Patients
CLINICAL PHARMACOLOGY: Clinical Studies
Concomitant Metformin and Insulin Therapy in Adult Patients
Specific Patient Populations
WARNINGS
HOW SUPPLIED
STORAGE AND HANDLING
SPL PATIENT PACKAGE INSERT
Metformin Hydrochloride Tablets, USPWhat is metformin?
What are the side effects of metformin?
Who should not take metformin?
-
● have kidney problems
-
● have liver problems
-
● have heart failure that is treated with medicines, such as Lanoxin(digoxin) or Lasix(furosemide)
-
● drink a lot of alcohol. This means you binge drink for short periods or drink all the time
-
● are seriously dehydrated (have lost a lot of water from your body)
-
● are going to have an x-ray procedure with injection of dyes (contrast agents)
-
● are going to have surgery
-
● develop a serious condition, such as heart attack, severe infection, or a stroke
-
● are 80 years or older and you have NOT had your kidney function tested
-
●
-
● Tell your doctor if you are pregnant or plan to become pregnant. Metformin may not be right for you. Talk with your doctor about your choices. You should also discuss your choices with your doctor if you are nursing a child.
How should I take metformin hydrochloride tablets?
-
● start to take other medicines or change how you take a medicine. Metformin can affect how well other drugs work, and some drugs can affect how well metformin work. Some medicines may cause high blood sugar.
-
●
-
● What should I avoid while taking metformin hydrochloride tablets?
What are the side effects of metformin?
-
● trouble breathing
-
● unusual or unexpected stomach discomfort
-
● feeling cold
-
● feeling dizzy or lightheaded
-
● suddenly developing a slow or irregular heartbeat
-
●
-
● If your medical condition suddenly changes, stop taking metformin and call your doctor right away. This may be a sign of lactic acidosis or another serious side effect.
General advice about prescription medicines
INACTIVE INGREDIENT
magnesium stearatemicrocrystalline cellulose
povidone
hypromellose
lactose monohydrate
red iron oxide
titanium dioxide
tricetin
vanillin
yellow iron oxide
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Metformin HydrochlorideMetformin Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!