3 Step First Aid Kit
3 Step First Aid Kit
FULL PRESCRIBING INFORMATION
Active ingredient
Drug fact
Antiseptic Swab:
Active Ingredient
Lidocaine 1% for pain relief
Benzalkonium Chloride 0.11% for antiseptic
Drug Fact
Povidone Iodine Swab:
Active Ingredient
Povidone Iodine 10%, equal to avilable Iodine 1% for antiseptic
Purpose
Uses
Keep out of reach of children
Uses
Direction
- Hold the swab vertically, with the color band tip upwards. Hold in the center of the stem with one hand and at teh color band with the other.
- Bend the tip at the color band to one side until it snaps.
- Apply the product to the affected area.
- Discard swab after use.
Warning
For external use only
Do not use
Stop use and ask a doctorif If
For adult and children 2 and older
Children under 2 years of age:
Antiseptic Swab: Dizolidinyl Urea, Methylparaben, Propylparaben, Water
Providone Iodine Swab: Glycerin, Water
Image of first aid carton label
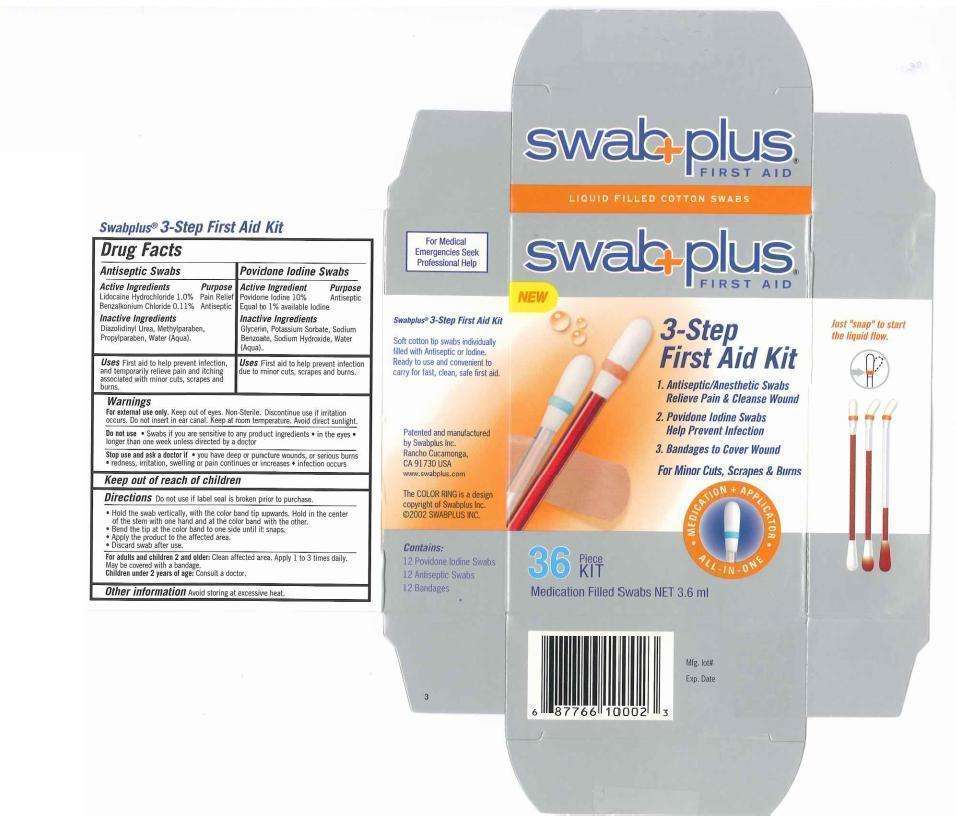
3 Step First Aid KitLidocaine Hydrochloride LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3-in-1 First Aid Povidone-Iodine SwabPovidone-Iodine LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||