Flavoxate Hydrochloride
Flavoxate Hydrochloride Tablets 100 mg, Film-CoatedRx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- FLAVOXATE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- FLAVOXATE HYDROCHLORIDE INDICATIONS AND USAGE
- FLAVOXATE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- FLAVOXATE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- FLAVOXATE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
FLAVOXATE HYDROCHLORIDE DESCRIPTION
Flavoxate hydrochloride tablets contain flavoxate hydrochloride, a synthetic urinary tract spasmolytic.
Chemically, flavoxate hydrochloride is 2-piperidinoethyl 3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylate hydrochloride. The empirical formula of flavoxate hydrochloride is C24H25NO4•HCl. The molecular weight is 427.94. The structural formula appears below.
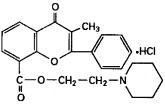
Each tablet for oral administration contains 100 mg flavoxate hydrochloride. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, corn starch, dibasic calcium phosphate dihydrate, hypromellose, polydextrose, magnesium stearate, titanium dioxide and triacetin.
CLINICAL PHARMACOLOGY
Flavoxate hydrochloride counteracts smooth muscle spasm of the urinary tract and exerts its effect directly on the muscle.
In a single study of 11 normal male subjects, the time to onset of action was 55 minutes. The peak effect was observed at 112 minutes. 57% of the flavoxate hydrochloride was excreted in the urine within 24 hours.
FLAVOXATE HYDROCHLORIDE INDICATIONS AND USAGE
Flavoxate hydrochloride tablets are indicated for symptomatic relief of dysuria, urgency, nocturia, suprapubic pain, frequency and incontinence as may occur in cystitis, prostatitis, urethritis, urethrocystitis/urethrotrigonitis. Flavoxate hydrochloride tablets are not indicated for definitive treatment, but are compatible with drugs used for the treatment of urinary tract infections.
FLAVOXATE HYDROCHLORIDE CONTRAINDICATIONS
Flavoxate hydrochloride is contraindicated in patients who have any of the following obstructive conditions: pyloric or duodenal obstruction, obstructive intestinal lesions or ileus, achalasia, gastrointestinal hemorrhage and obstructive uropathies of the lower urinary tract.
WARNINGS
Flavoxate hydrochloride should be given cautiously in patients with suspected glaucoma.
PRECAUTIONS
Information for Patients
Patients should be informed that if drowsiness and blurred vision occur, they should not operate a motor vehicle or machinery or participate in activities where alertness is required.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenicity studies and long-term studies in animals to determine the carcinogenic potential of flavoxate hydrochloride have not been performed.
Pregnancy
Reproduction studies have been performed in rats and rabbits at doses up to 34 times the human dose and revealed no evidence of impaired fertility or harm to the fetus due to flavoxate hydrochloride. There are, however, no well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when flavoxate hydrochloride is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children below the age of 12 years have not been established.
FLAVOXATE HYDROCHLORIDE ADVERSE REACTIONS
The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency.
Gastrointestinal: Nausea, vomiting, dry mouth.
CNS: Vertigo, headache, mental confusion, especially in the elderly, drowsiness, nervousness.
Hematologic: Leukopenia (one case which was reversible upon discontinuation of the drug).
Cardiovascular: Tachycardia and palpitation.
Allergic: Urticaria and other dermatoses, eosinophilia and hyperpyrexia.
Ophthalmic: Increased ocular tension, blurred vision, disturbance in eye accommodation.
Renal: Dysuria.
OVERDOSAGE
The oral LD50 for flavoxate hydrochloride in rats is 4273 mg/kg. The oral LD50 for flavoxate hydrochloride in mice is 1837 mg/kg.
It is not known whether flavoxate hydrochloride is dialyzable.
FLAVOXATE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Adults and children over 12 years of age
One or two 100 mg tablets 3 or 4 times a day. With improvement of symptoms, the dose may be reduced. This drug cannot be recommended for infants and children under 12 years of age because safety and efficacy have not been demonstrated in this age group.
HOW SUPPLIED
Flavoxate hydrochloride 100 mg tablets are available as white, round biconvex, film-coated tablets, debossed “Є 58” on one side and plain on the other side.
Store at 20° - 25°C (68° - 77°F) [see USP Controlled Room Temperature].
NDC 50268-324-15
10 tablets per card, 5 cards per carton
Dispensed in a blister punch material for Institutional Use Only.
Manufactured for:
AvPAK
A Product of AvKARE
Pulaski, TN 38478
Mfg. Iss. 01/11
AV. Rev. 12/12 ( P)
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 50268-324-15
Flavoxate Hydrochloride Tablets
100 mg
Rx Only
50 Tablets (5 X 10) Unit Dose
5026832415
NDC 50268-324-15
Flavoxate Hydrochloride Tablets
100 mg
Rx Only
50 Tablets (5 X 10) Unit Dose
5026832415
Each film-coated tablet contains:
Flavoxate Hydrochloride...............................100 mg
USUAL DOSAGE: See accompanying literature for complete prescribing information.
Store at 20o-25oC (68o-77oF) [see USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
AvPAK
A PRODUCT OF AvKARE
Mfg. Iss. 08/10 AV 12/12 (P)
Flavoxate HydrochlorideFlavoxate Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||