Folic Acid
Folic Acid Injection, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- FOLIC ACID DESCRIPTION
- CLINICAL PHARMACOLOGY
- FOLIC ACID INDICATIONS AND USAGE
- WARNINGS
- PRECAUTIONS
- FOLIC ACID ADVERSE REACTIONS
- FOLIC ACID DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
FOLIC ACID DESCRIPTION
Folic Acid Injection, USP is a sterile, nonpyrogenic solution of sodium folate (prepared by the addition of sodium hydroxide to folic acid) in Water for Injection intended for intramuscular (IM), intravenous (IV) or subcutaneous (SC) use.
Folic Acid is a complex organic compound present in liver, yeast and other substances, which may be prepared synthetically. It is a yellow or yellowish orange, odorless crystalline powder. It is very slightly soluble in water, insoluble in alcohol, chloroform, ether; readily dissolves in dilute solutions of alkali hydroxides and carbonates. It is chemically designated as: L-Glutamic acid, N -[4-[[(2-amino-1-4-dihydro-4-oxo-6-pteridinyl) methyl] amino]benzoyl]-, and has the following structural formula.
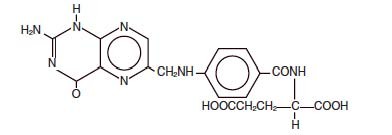
C19H19N7O6 M.W. 441.40
Each mL contains: Sodium folate (equivalent to 5 mg folic acid); edetate disodium 2 mg; benzyl alcohol 15 mg (added as preservative); Water for Injection q.s. Hydrochloric acid and/or sodium hydroxide for pH adjustment (8.0 to 11.0).
CLINICAL PHARMACOLOGY
In man, an exogenous source of folate is required for nucleoprotein synthesis and maintenance of normal erythropoiesis. Folic acid, whether given by mouth or parenterally, stimulates specifically the production of red blood cells, white blood cells and platelets in persons suffering from certain megaloblastic anemias.
FOLIC ACID INDICATIONS AND USAGE
Folic Acid Injection, USP alone is effective in the treatment of megaloblastic anemias due to a deficiency of folic acid as may be seen in tropical or nontropical sprue, in anemias of nutritional origin, pregnancy, infancy or childhood.
WARNINGS
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient.
This product contains benzyl alcohol. Benzyl alcohol has been reported to be associated with a fatal “Gasping Syndrome” in premature infants.
PRECAUTIONS
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
FOLIC ACID ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
FOLIC ACID DOSAGE AND ADMINISTRATION
Parenteral Administration : IM, IV and SC routes may be used if the disease is exceptionally severe or if gastrointestinal absorption may be, or is known to be, impaired.
Usual Therapeutic Dosage — In adults and children (regardless of age): up to 1 mg daily.Resistant cases may require larger doses.
Maintenance Level : When clinical symptoms have subsided and the blood picture has become normal, a maintenance level should be used, i.e., 0.1 mg for infants and up to 0.3 mg for children under four years of age, 0.4 mg for adults and children four or more years of age and 0.8 mg for pregnant and lactating women, per day, but never less than 0.1 mg per day. Patient should be kept under close supervision and adjustment of the maintenance level made if relapse appears imminent.
In the presence of alcoholism, hemolytic anemia, anticonvulsant therapy or chronic infection, the maintenance level may need to be increased.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
Folic Acid Injection, USP (5 mg/mL) is available as:
|
Product No. |
NDC No. |
|
|
18410 |
63323-184-10 |
10 mL Multiple Dose, in a flip-top vial packaged individually. |
|
18411 |
63323-184-11 |
10 mL Multiple Dose, in a flip-top vial, 10 vials per tray. |
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
PROTECT FROM LIGHT.
Retain vial in carton until contents are used.

45842F
Revised: October 2010
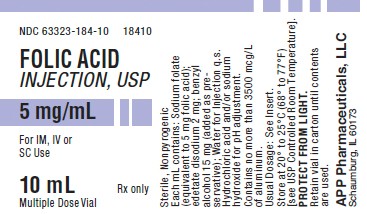
PACKAGE LABEL - PRINCIPAL DISPLAY - Folic Acid 10 mL Vial Label
NDC 63323-184-10
18410
Folic Acid Injection, USP
5 mg/mL
For IM, IV, or SC Use
10 mL
Rx only
Multiple Dose Vial
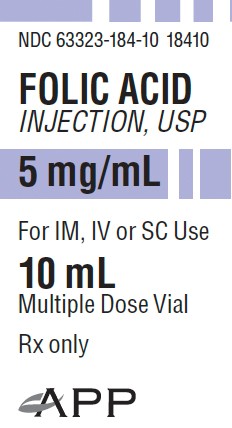
PACKAGE LABEL - PRINCIPAL DISPLAY - Folic Acid 10 mL Vial Carton Label
NDC 63323-184-10
18410
Folic Acid Injection, USP
5 mg/mL
For IM, IV, or SC Use
10 mL
Multiple Dose Vial
Rx only
Folic AcidFolic Acid INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||