Pantoprazole Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- PANTOPRAZOLE SODIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- USE IN SPECIFIC POPULATIONS
- PHARMACODYNAMICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- PANTOPRAZOLE SODIUM CONTRAINDICATIONS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- PANTOPRAZOLE SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PANTOPRAZOLE SODIUM DESCRIPTION
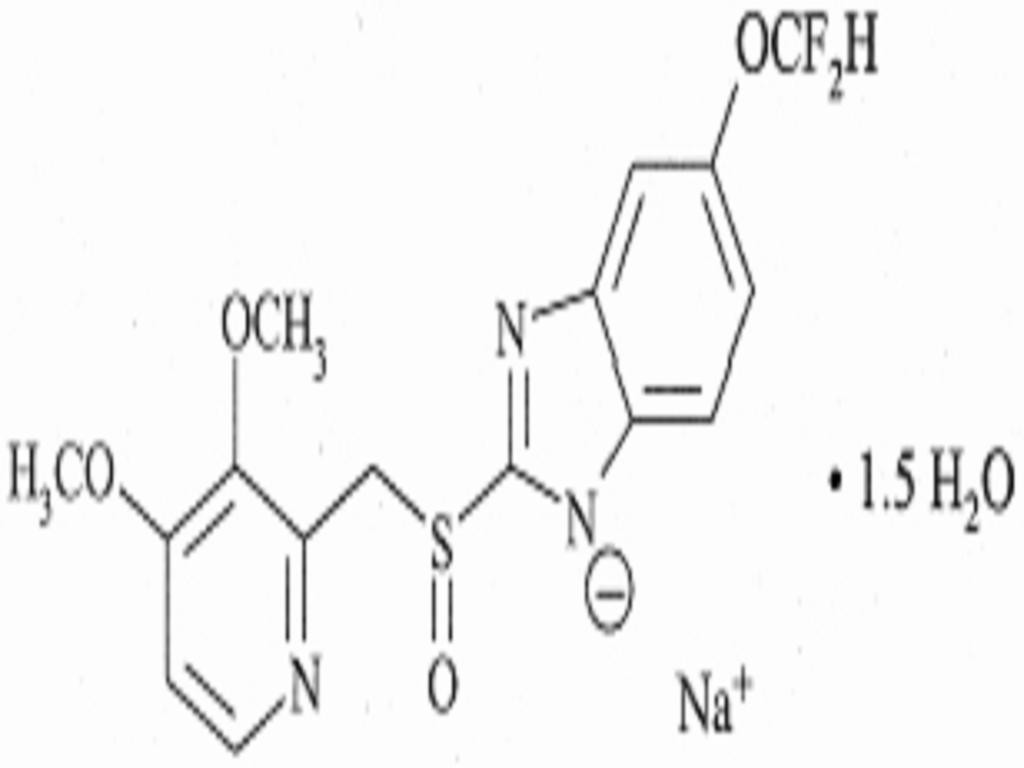
CLINICAL PHARMACOLOGY
Pharmacokinetics
CLINICAL PHARMACOLOGY, Pharmacokinetics, Metabolism
Absorption
Distribution
Metabolism
Elimination
USE IN SPECIFIC POPULATIONS
GeriatricPediatric
Gender
Renal Impairment
Hepatic Impairment
Drug-Drug Interactions
PHARMACODYNAMICS
Mechanism of Action
Antisecretory Activity
Serum Gastrin Effects
Enterochromaffin-Like (ECL) Cell Effects
Other Effects
CLINICAL STUDIES
Erosive Esophagitis (EE) Associated with Gastroesophageal Reflux Disease (GERD)
Long-Term Maintenance of Healing of Erosive Esophagitis
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
INDICATIONS & USAGE
Short-Term Treatment of Erosive Esophagitis Associated With Gastroesophageal Reflux Disease (GERD)Maintenance of Healing of Erosive Esophagitis
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
PANTOPRAZOLE SODIUM CONTRAINDICATIONS
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category B
NURSING MOTHERS
PEDIATRIC USE
Use in Women
GERIATRIC USE
Laboratory Tests
PANTOPRAZOLE SODIUM ADVERSE REACTIONS
Postmarketing Reports
Laboratory Values
OVERDOSAGE
DOSAGE & ADMINISTRATION
Treatment of Erosive EsophagitisMaintenance of Healing of Erosive Esophagitis
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
Patient Counseling
-
● Caution patients that pantoprazole sodium delayed-release tablets, should not be split, crushed, or chewed.
-
● Tell patients that pantoprazole sodium delayed-release tablets, USP should be swallowed whole, with or without food in the stomach.
-
● Let patients know that concomitant administration of antacids does not affect the absorption of pantoprazole sodium delayed-release tablets, USP.
PATIENT INFORMATION
What is pantoprazole sodium?
-
● Up to 8 weeks for short-term treatment of acid-related damage to the lining of the esophagus (erosive esophagitis) caused by gastroesophageal reflux disease (GERD). If needed, your doctor may prescribe an additional 8 weeks of pantoprazole sodium delayed-release tablets.
-
● Maintain healing of acid-related damage to the lining of the esophagus and helps prevent return of heartburn symptoms caused by GERD. Pantoprazole sodium delayed-release tablets have not been studied for treatment lasting longer than 1 year
-
● Treating a rare condition called Zollinger-Ellison Syndrome, where the stomach makes more than the normal amount of acid
Who should not take pantoprazole sodium delayed-release tablets, USP?
-
● allergic to any of the ingredients in pantoprazole sodium delayed-release tablets. See the end of this leaflet for a complete list of ingredients in pantoprazole sodium delayed-release tablets.
-
● allergic to any proton pump inhibitor (PPI). If you do not know if your medicines are PPIs, please ask your doctor.
Before taking pantoprazole sodiumdelayed-release tablets, tell your doctor about all your medical conditions, including if you are:
-
● pregnant, think you may be pregnant, or are planning to become pregnant. It is not known if pantoprazole sodium delayed-release tablets will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
-
● breastfeeding or planning to breastfeed. Pantoprazole sodium delayed-release tablets may pass into your milk. Talk with your doctor about the best way to feed your baby if you take pantoprazole sodium delayed-release tablets.
-
● Warfarin (CoumadinAthrombin -KJantovenPanwarfin
-
● Ketoconazole (Nizoral
-
● Atazanavir (ReyatazNelfinavir (Viracept
-
● Iron supplements
-
● Ampicillin antibiotics
How should I take pantoprazole sodium delayed-release tablets, USP?
-
● Take pantoprazole sodium delayed-release tablets exactly as prescribed by your doctor.
-
● Do not change your dose or stop pantoprazole sodium delayed-release tablets without talking to your doctor.
-
● If you forget to take a dose of pantoprazole sodium delayed-release tablets, take it as soon as you remember. If it is almost time for your next dose, do not take the missed dose. Take the next dose at your regular time. Do not take two doses to try to make up for a missed dose.
-
● If you take too much pantoprazole sodium delayed-release tablets, call your doctor right away.
-
● See the Patient Instructions for Use at the end of this leaflet for detailed instructions about:
-
● how to take pantoprazole sodium delayed-release tablets .
-
● Stomach lining weakening with long-term use
-
● Vitamin B-12 deficiency
-
● Serious allergic reactions. Tell your doctor if you get any of the following symptoms with pantoprazole sodium delayed-release tablets
-
● rash
-
● face swelling
-
● throat tightness
-
● difficult breathing
How should I store pantoprazole sodium delayed-release tablets, USP?
-
● Store pantoprazole sodium delayed-release tablets at 20(68
-
● Keep pantoprazole sodium delayed-release tablets and all medicines out of the reach of children.
What are the ingredients in pantoprazole sodium delayed-release tablets, USP?
Active ingredient:
Inactive ingredients in pantoprazole sodium delayed-release tablets, USP:
Patient Instructions for Use
-
● You can take pantoprazole sodium delayed-release tablets with food or on an empty stomach.
-
● Swallow pantoprazole sodium delayed-release tablets whole.
-
● If you have trouble swallowing a pantoprazole sodium delayed-release tablet 40 mg tablet, you can take two 20 mg tablets instead.
-
● Do not split, chew or crush pantoprazole sodium delayed-release tablets.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Pantoprazole SodiumPantoprazole Sodium TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!