Sumadan Wash
Medimetriks Pharmaceuticals inc.
Sumadan (Sodium Sulfacetamide 9% & Sulfur 4.5%) WASH
FULL PRESCRIBING INFORMATION: CONTENTS*
- SUMADAN WASH DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS
- SUMADAN WASH CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- SUMADAN WASH ADVERSE REACTIONS:
- SUMADAN WASH DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 454 g Bottle Carton
- PRINCIPAL DISPLAY PANEL - 454 g Bottle Carton
FULL PRESCRIBING INFORMATION
Rx Only
In a Moisturizing
Novasome® Vehicle
SUMADAN WASH DESCRIPTION
Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Chemically sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
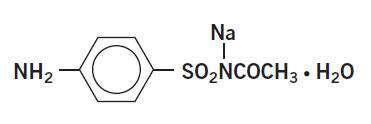
Each mL of Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash contains 90 mg of sodium sulfacetamide and 45 mg of sulfur in a formulation consisting of: butylated hydroxytoluene, C12-15 alkyl benzoate, caprylyl glycol, cetyl alcohol, cholesterol, chrysanthemum dendranthema, dimethicone, disodium oleamido MIPA sulfosuccinate, edetate disodium, ethylene brassilate, glyceryl stearate, hexylene glycol, lemon oil, magnesium aluminum silicate, magnesium chloride, magnesium nitrate, methylchloroisothiazolinone, methylisothiazolinone, niacinamide, nonoxynol-20, octoxynol-5, purified water, PEG-100 stearate, phenoxyethanol, propylene glycol, sodium cocoyl isotheionite, sodium methyl cocoyl taurate, sodium thiosulfate, stearyl alcohol, xanthan gum.
CLINICAL PHARMACOLOGY
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (PABA), an essential component for bacterial growth. While absorption through intact skin has not been determined, sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine, largely unchanged. The biological half-life has variously been reported as 7 to 12.8 hours. The exact mode of action of sulfur in the treatment of acne is unknown, but it has been reported that it inhibits the growth of Propionibacterium acnes and the formation of free fatty acids.
INDICATIONS
Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash is indicated for the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
SUMADAN WASH CONTRAINDICATIONS
Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash are contraindicated for use by patients having known hypersensitivity to sulfonamides, sulfur or any other component of this preparation. Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash is not to be used by patients with kidney disease.
WARNINGS
Although rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to hypersensitivity to topical sulfonamides. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice, and contact dermatitis indicate hypersensitivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved.
FOR EXTERNAL USE ONLY. Keep away from eyes. Keep out of reach of children. Keep container tightly closed.
PRECAUTIONS
General
If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or sensitization during long-term therapy. The object of this therapy is to achieve desquamation without irritation, but sodium sulfacetamide and sulfur can cause reddening and scaling of the epidermis. These side effects are not unusual in the treatment of acne vulgaris, but patients should be cautioned about the possibility.
Information for Patients
Avoid contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. If excessive irritation develops, discontinue use and consult your physician.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
PREGNANCY
Category C
Animal reproduction studies have not been conducted with Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash. It is also not known whether Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether sodium sulfacetamide is excreted in the human milk following topical use of Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash. However, small amounts of orally administered sulfonamides have been reported to be eliminated in human milk. In view of this and because many drugs are excreted in human milk, caution should be exercised when Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash is administered to a nursing woman.
PEDIATRIC USE
Safety and effectiveness in children under the age of 12 have not been established.
ADVERSE REACTIONS:
Although rare, sodium sulfacetamide may cause local irritation.
SUMADAN WASH DOSAGE AND ADMINISTRATION
Apply Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash once or twice daily to affected areas, or as directed by your physician. Wet skin and liberally apply to areas to be cleansed. Massage gently into skin for 10-20 seconds, working into a full lather, rinse thoroughly and pat dry. If drying occurs, it may be controlled by rinsing off Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash sooner or using less often.
HOW SUPPLIED
Sumadan® (sodium sulfacetamide 9% & sulfur 4.5%) Wash is available in a 16 fl. oz. (473 mL) bottle, NDC 43538-190-16.
Store at controlled room temperature 15°- 30° C (59°-86° F). Protect from freezing.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured for:
MEDIMETRIKS
PHARMACEUTICALS, INC.
383 Route 46 West
Fairfield, NJ 07004-2402 USA
www.medimetriks.com
IP022-R2
Rev. 3/13
PRINCIPAL DISPLAY PANEL - 454 g Bottle Carton
NDC 43538-190-16
Rx Only
Sumadan™
(Sodium Sulfacetamide 9% & Sulfur 4.5%)
WASH
In a moisturizing
Novasome® vehicle
Net Wt. 16 oz. (454 g)
MEDIMETRIKS
PHARMACEUTICALS, INC.
PRINCIPAL DISPLAY PANEL - 454 g Bottle Carton
NDC 43538-191-16
Rx Only
Sumadan™
(Sodium Sulfacetamide 9% & Sulfur 4.5%)
KIT
CONTENTS:
1 - Sumadan™ (Sodium Sulfacetamide 9% & Sulfur 4.5%) Wash (Net Wt. 16 oz.)
1- Rehyla™ Wash Moisturizing Daily Wash (16 fl. oz.)
MEDIMETRIKS
PHARMACEUTICALS, INC.

Sumadan Washsulfacetamide sodium and sulfur CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sumadansulfacetamide sodium and sulfur KIT
| ||||||||||||||||||||||||||||||||||||||||