Bupropion Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING SECTION
- BUPROPION HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- BUPROPION HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- BUPROPION HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL MEDGUIDE
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BUPROPION HYDROCHLORIDE DESCRIPTION
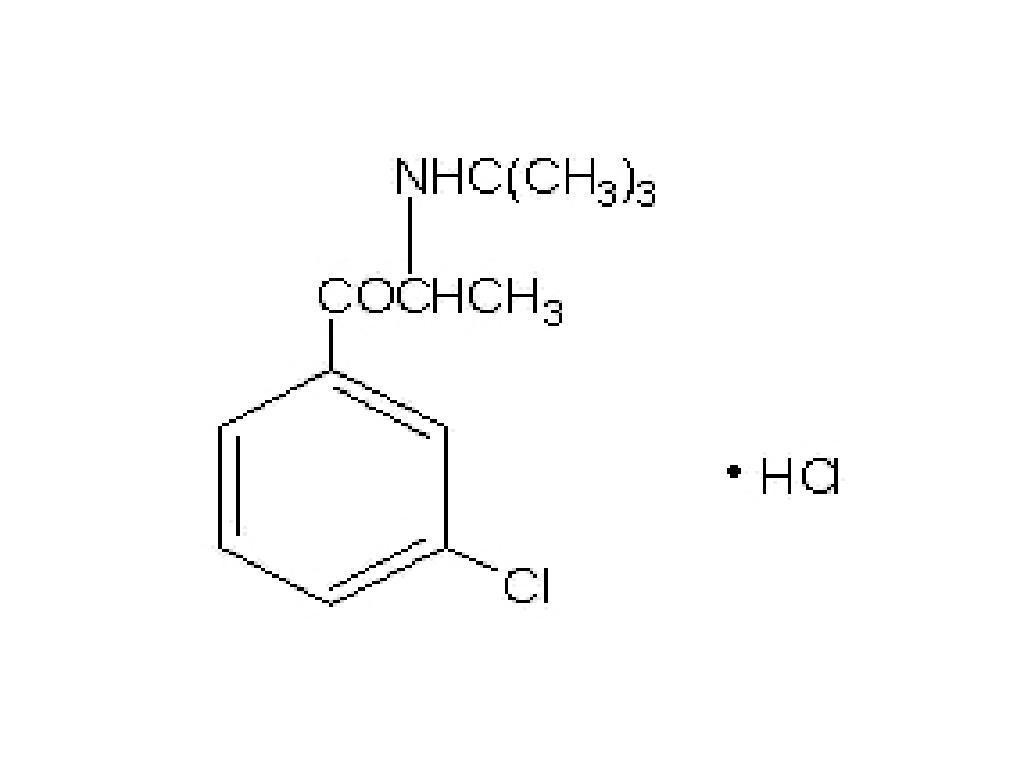
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
BUPROPION HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
BUPROPION HYDROCHLORIDE ADVERSE REACTIONS
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
DOSAGE & ADMINISTRATION
HOW SUPPLIED
SPL MEDGUIDE
INACTIVE INGREDIENT
ANHYDROUS LACTOSESILICON DIOXIDE
CROSPOVIDONE
HYDROCHLORIC ACID
HYPROMELLOSE
CELLULOSE, MICROCRYSTALLINE
POLYDEXTROSE
POLYETHYLENE GLYCOL
STEARIC ACID
TITANIUM DIOXIDE
TRIACETIN
FD&C BLUE NO. 2
FD&C YELLOW NO. 6
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Bupropion HydrochlorideBupropion Hydrochloride TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!