Ciprofloxacin
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- CLINICAL PHARMACOLOGY
- CIPROFLOXACIN DESCRIPTION
- INDICATIONS & USAGE
- MICROBIOLOGY
- CIPROFLOXACIN CONTRAINDICATIONS
- CIPROFLOXACIN ADVERSE REACTIONS
- WARNINGS AND PRECAUTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- CLINICAL STUDIES
- REFERENCES
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
CLINICAL PHARMACOLOGY
Absorption:Dose (mg)Maximum Serum Concentration (Area Under Curve (AUC) (hr/mL)
Distribution:
Metabolism:
CONTRAINDICATIONSWARNINGSPRECAUTIONS: Drug Interactions
Excretion:
Drug-drug Interactions:
PRECAUTIONS
CONTRAINDICATIONS
WARNINGS:PRECAUTIONS
Special Populations:
PRECAUTIONS: Geriatric Use
DOSAGE AND ADMINISTRATION
CIPROFLOXACIN DESCRIPTION

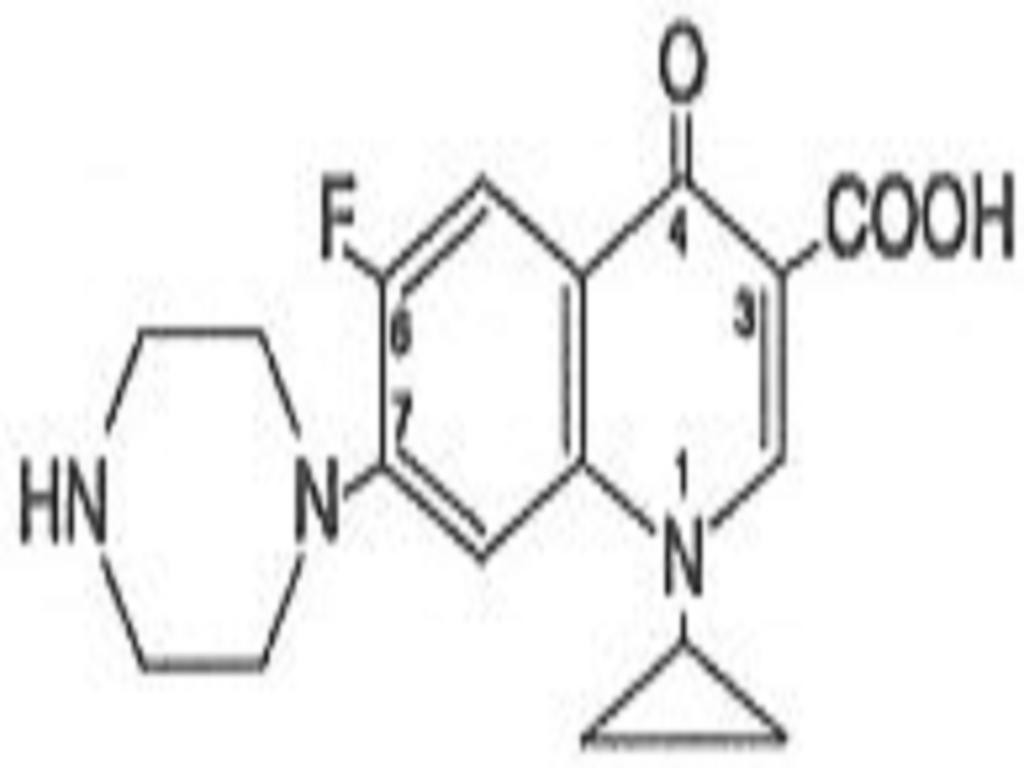
INDICATIONS & USAGE
DOSAGE AND ADMINISTRATIONAdult Patients:
Pediatric patients (1 to 17 years of age):
WARNINGS,PRECAUTIONS, Pediatric Use,ADVERSE REACTIONSCLINICAL STUDIESANIMAL PHARMACOLOGY
Adult and Pediatric Patients:
INHALATIONAL ANTHRAXADDITIONAL INFORMATION
MICROBIOLOGY
INDICATIONS AND USAGE
INDICATIONS AND USAGEINHALATIONAL ANTHRAXADDITIONAL INFORMATION
Susceptibility Tests
Dilution Techniques:
**
Diffusion Techniques:
CIPROFLOXACIN CONTRAINDICATIONS
PRECAUTIONS: Drug Interactions
CIPROFLOXACIN ADVERSE REACTIONS
Adverse Reactions in Adult Patients:Adverse Reactions in Pediatric Patients:
Post-Marketing Adverse Event Reports:
PRECAUTIONS
INHALATIONAL ANTHRAX - ADDITIONAL INFORMATION
WARNINGS AND PRECAUTIONS
Tendinopathy and Tendon RupturePregnant Women:
PRECAUTIONS: Pregnancy,Nursing Mothers
Pediatrics:
INDICATIONS AND USAGEADVERSE REACTIONS
ANIMAL PHARMACOLOGY
Cytochrome P450 (CYP450):
Central Nervous System Disorders:
PRECAUTIONS: GeneralInformation for PatientsDrug InteractionsADVERSE REACTIONS.
Theophylline:
Hypersensitivity Reactions:
-
●
-
● vasculitis; arthralgia; myalgia; serum sickness;
-
● allergic pneumonitis;
-
● interstitial nephritis; acute renal insufficiency or failure;
-
● hepatitis; jaundice; acute hepatic necrosis or failure;
-
● anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
-
● The drug should be discontinued immediately at the first appearance of a skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted (SeePRECAUTIONS: Information for PatientsandADVERSE REACTIONS).
-
●
Peripheral neuropathy:
Syphilis:
PRECAUTIONS
General:
ANIMAL PHARMACOLOGY
Central Nervous System:
WARNINGSInformation for Patients,Drug Interactions
Renal Impairment:
DOSAGE AND ADMINISTRATION
Photosensitivity/Phototoxicity:
Information for Patients:
-
● that ciprofloxacin may be taken with or without meals and to drink fluids liberally. As with other quinolones, concurrent administration of ciprofloxacin with magnesium/aluminum antacids, or sucralfate, Videx
-
● that ciprofloxacin may be associated with hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash or other allergic reaction.
-
● that photosensitivity/phototoxicity has been reported in patients receiving quinolones. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking quinolones. If patients need to be outdoors while using quinolones, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, patients should contact their physician.
-
● that peripheral neuropathies have been associated with ciprofloxacin use. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, they should discontinue treatment and contact their physicians.
-
● that ciprofloxacin may cause dizziness and lightheadedness; therefore, patients should know how they react to this drug before they operate an automobile or machinery or engage in activities requiring mental alertness or coordination.
-
● that ciprofloxacin increases the effects of tizanidine (ZanaflexPatients should not use ciprofloxacin if they are already taking tizanidine.
-
● that ciprofloxacin may increase the effects of theophylline and caffeine. There is a possibility of caffeine accumulation when products containing caffeine are consumed while taking quinolones.
-
● that convulsions have been reported in patients receiving quinolones, including ciprofloxacin, and to notify their physician before taking this drug if there is a history of this condition.
-
● that ciprofloxacin has been associated with an increased rate of adverse events involving joints and surrounding tissue structures (like tendons) in pediatric patients (less than 18 years of age). Parents should inform their child's physician if the child has a history of joint-related problems before taking this drug. Parents of pediatric patients should also notify their child's physician of any joint-related problems that occur during or following ciprofloxacin therapy. (SeeWARNINGS,PRECAUTIONS, Pediatric UseandADVERSE REACTIONS.)
-
● that diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
-
●
-
● Drug Interactions:
WARNINGS
DOSAGE AND ADMINISTRATION
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Pregnancy: Teratogenic Effects. Pregnancy Category C:
WARNINGS
WARNINGS
Nursing Mothers:
Pediatric Use:
ANIMAL PHARMACOLOGY
DOSAGE AND ADMINISTRATIONINHALATIONAL ANTHRAXADDITIONAL INFORMATION
ADVERSE REACTIONSCLINICAL STUDIES
Geriatric Use:
Boxed Warning,WARNINGS, and ADVERSE REACTIONS/Post-Marketing Adverse Event Reports).
In a retrospective analysis of 23 multiple-dose controlled clinical trials of ciprofloxacin encompassing over 3500 ciprofloxacin treated patients, 25% of patients were greater than or equal to 65 years of age and 10% were greater than or equal to 75 years of age. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals on any drug therapy cannot be ruled out. Ciprofloxacin is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. No alteration of dosage is necessary for patients greater than 65 years of age with normal renal function. However, since some older individuals experience reduced renal function by virtue of their advanced age, care should be taken in dose selection for elderly patients, and renal function monitoring may be useful in these patients. (SeeCLINICAL PHARMACOLOGYandDOSAGE AND ADMINISTRATION.)
In general, elderly patients may be more susceptible to drug-associated effects on the QT interval. Therefore, precaution should be taken when using ciprofloxacin hydrochloride with concomitant drugs that can result in prolongation of the QT interval (e.g., class IA or class III antiarrhythmics) or in patients with risk factors for torsade de pointes (e.g., known QT prolongation, uncorrected hypokalemia).
OVERDOSAGE
DOSAGE AND ADMINISTRATION - ADULTS
Dosage Guidelines table
InfectionSeverityDoseFrequencyUsual Durations
INHALATIONAL ANTHRAXADDITIONAL INFORMATION
Conversion of I.V. to Oral Dosing in Adults:
CLINICAL PHARMACOLOGY
Adults with Impaired Renal Function:
DOSAGE & ADMINISTRATION
Dosage Guidelines tableADVERSE REACTIONSCLINICAL STUDIESINHALATIONAL ANTHRAXADDITIONAL INFORMATION
HOW SUPPLIED
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
WARNINGSCLINICAL STUDIES
Complicated Urinary Tract Infection and PyelonephritisEfficacy in Pediatric Patients:
CiprofloxacinComparatorEscherichia coli
INHALATIONAL ANTHRAX IN ADULTS AND PEDIATRICSADDITIONAL INFORMATION
DOSAGE AND ADMINISTRATIONPRECAUTIONS, Pediatric Use
REFERENCES
SPL MEDGUIDE
-
● Tendons are tough cords of tissue that connect muscles to bones.
-
● Pain, swelling, tears and inflammation of tendons including the back of the ankle (Achilles), shoulder, hand, or other tendon sites can happen in people of all ages who take fluoroquinolone antibiotics, including ciprofloxacin hydrochloride tablets. The risk of getting tendon problems is higher if you:
-
● are over 60 years of age
-
● are taking steroids (corticosteroids)
-
● have had a kidney, heart or lung transplant.
-
● Swelling of the tendon (tendinitis) and tendon rupture (breakage) have also happened in patients who take fluoroquinolones who do not have the above risk factors.
-
● Other reasons for tendon ruptures can include:
-
● physical activity or exercise
-
● kidney failure
-
● tendon problems in the past, such as in people with rheumatoid arthritis (RA)
-
● Call your healthcare provider right away at the first sign of tendon pain, swelling or inflammation. Stop taking ciprofloxacin hydrochloride tablets until tendinitis or tendon rupture has been ruled out by your healthcare provider. Avoid exercise and using the affected area. The most common area of pain and swelling is the Achilles tendon at the back of your ankle. This can also happen with other tendons. Talk to your healthcare provider about the risk of tendon rupture with continued use of ciprofloxacin hydrochloride tablets. You may need a different antibiotic that is not a fluoroquinolone to treat your infection.
-
● Tendon rupture can happen while you are taking or after you have finished taking ciprofloxacin hydrochloride tablets. Tendon ruptures have happened up to several months after patients have finished taking their fluoroquinolone.
-
● Get medical help right away if you get any of the following signs or symptoms of a tendon rupture:
-
● hear or feel a snap or pop in a tendon area
-
● bruising right after an injury in a tendon area
-
● unable to move the affected area or bear weight
-
● See the sectionWhat are the possible side effects of ciprofloxacin hydrochloride tablets?for more information about side effects.
-
● What are ciprofloxacin hydrochloride tablets?
-
● Ciprofloxacin hydrochloride tablets are fluoroquinolone antibiotic medicine used to treat certain infections caused by certain germs called bacteria.
-
● What should I tell my healthcare provider before taking ciprofloxacin hydrochloride tablets?
-
● SeeWhat is the most important information I should know about ciprofloxacin hydrochloride tablets?
-
● have nerve problems
-
● have or anyone in your family has an irregular heartbeat, especially a condition calledQT prolongation
-
● have a history of seizures
-
● have kidney problems. You may need a lower dose of ciprofloxacin hydrochloride tablets if your kidneys do not work well.
-
● have rheumatoid arthritis (RA) or other history of joint problems
-
● have trouble swallowing pills
-
● are pregnant or planning to become pregnant. It is not known if ciprofloxacin hydrochloride tablets will harm your unborn child.
-
● are breast-feeding or planning to breast-feed. Ciprofloxacin hydrochloride tablets passes into breast milk. You and your healthcare provider should decide whether you will take ciprofloxacin hydrochloride tablets or breast-feed.
-
● Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal and dietary supplements. Ciprofloxacin hydrochloride tablets and other medicines can affect each other causing side effects. Especially tell your healthcare provider if you take:
-
● an NSAID (Non-Steroidal Anti-Inflammatory Drug). Many common medicines for pain relief are NSAIDs. Taking an NSAID while you take ciprofloxacin hydrochloride tablets or other fluoroquinolones may increase your risk of central nervous system effects and seizures. SeeWhat are the possible side effects of ciprofloxacin hydrochloride tablets?.
-
● tizanidine (ZanaflexYou should not take ciprofloxacin hydrochloride tablets if you are already taking tizanidine. SeeWho should not take ciprofloxacin hydrochloride tablets?
-
● theophylline (Theo-24ElixophyllinTheochronUniphylTheolair
-
● glyburide (MicronaseGlynaseDiabetaGlucovanceSeeWhat are the possible side effects of ciprofloxacin hydrochloride tablets?
-
● phenytoin (Fosphenytoin SodiumCerebyxDilantin-125DilantinExtended Phenytoin SodiumPrompt Penytoin SodiumPhenytek
-
● products that contain caffeine
-
● a medicine to control your heart rate or rhythm (antiarrhythmics) SeeWhat are the possible side effects of ciprofloxacin hydrochloride tablets?
-
● an anti-psychotic medicine
-
● a tricyclic antidepressant
-
● a water pill (diuretic)
-
● a steroid medicine. Corticosteroids taken by mouth or by injection may increase the chance of tendon injury. SeeWhat is the most important information I should know about ciprofloxacin hydrochloride tablets?
-
● methotrexate (Trexall
-
● Probenecid (ProbalanCol-probenecid
-
● Metoclopromide (ReglanReglan ODT
-
● Certain medicines may keep ciprofloxacin hydrochloride tablets from working correctly. Take ciprofloxacin hydrochloride tablets either 2 hours before or 6 hours after taking these products:
-
● an antacid, multivitamin, or other product that has magnesium, calcium, aluminum, iron, or zinc
-
● sucralfate (Carafate
-
● didanosine (VidexVidexEC).
-
● Ask your healthcare provider if you are not sure if any of your medicines are listed above.
-
● Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
-
● Ciprofloxacin hydrochloride tablets can be taken with or without food.
-
● Ciprofloxacin hydrochloride tablets should not be taken with dairy products (like milk or yogurt) or calcium-fortified juices alone, but may be taken with a meal that contains these products.
-
● Drink plenty of fluids while taking ciprofloxacin hydrochloride tablets.
-
● Do not skip any doses, or stop taking ciprofloxacin hydrochloride tablets even if you begin to feel better, until you finish your prescribed treatment, unless:
-
● you have tendon effects (seeWhat is the most important information I should know about ciprofloxacin hydrochloride tablets?),
-
● you have a serious allergic reaction (seeWhat are the possible side effects of ciprofloxacin hydrochloride tablets?), or your healthcare provider tells you to stop.
-
● This will help make sure that all of the bacteria are killed and lower the chance that the bacteria will become resistant to ciprofloxacin hydrochloride tablets. If this happens, ciprofloxacin hydrochloride tablets and other antibiotic medicines may not work in the future.
-
● If you miss a dose of ciprofloxacin hydrochloride tablets, take it as soon as you remember. Do not take two doses at the same time, and do not take more than two doses in one day.
-
● If you take too much, call your healthcare provider or get medical help immediately.
-
● If you have been prescribed ciprofloxacin hydrochloride tablets after being exposed to anthrax:
-
● Ciprofloxacin hydrochloride tablets have been approved to lessen the chance of getting anthrax disease or worsening of the disease after you are exposed to the anthrax bacteria germ.
-
● Side effects may happen while you are taking ciprofloxacin hydrochloride tablets. When taking your ciprofloxacin hydrochloride tablets to prevent anthrax infection, you and your healthcare provider should talk about whether the risks of stopping ciprofloxacin hydrochloride tablets too soon are more important than the risks of side effects with ciprofloxacin hydrochloride tablets.
-
● If you are pregnant, or plan to become pregnant while taking ciprofloxacin hydrochloride tablets, you and your healthcare provider should decide whether the benefits of taking ciprofloxacin hydrochloride tablets for anthrax are more important than the risks.
-
● What should I avoid while taking ciprofloxacin hydrochloride tablets?
-
● Ciprofloxacin hydrochloride tablets can make you feel dizzy and lightheaded. Do not drive, operate machinery, or do other activities that require mental alertness or coordination until you know how ciprofloxacin hydrochloride tablets affect you.
-
● What are the possible side effects of ciprofloxacin hydrochloride tablets?
-
● Ciprofloxacin hydrochloride tablets can cause side effects that may be serious or even cause death. SeeWhat is the most important information I should know about ciprofloxacin hydrochloride tablets?Other serious side effects of ciprofloxacin hydrochloride tablets include:
-
● feel dizzy
-
● seizures
-
● hear voices, see things, or sense things that are not there (hallucinations)
-
● feel restless
-
● tremors
-
● feel anxious or nervous
-
● confusion
-
● depression
-
● trouble sleeping
-
● nightmares
-
● feel more suspicious (paranoia)
-
● suicidal thoughts or acts
-
● Serious allergic reactions
-
● Allergic reactions can happen in people taking fluoroquinolones, including ciprofloxacin hydrochloride tablets, even after only one dose. Stop taking ciprofloxacin hydrochloride tablets and get emergency medical help right away if you get any of the following symptoms of a severe allergic reaction:
-
● hives
-
● trouble breathing or swallowing
-
● swelling of the lips, tongue, face
-
● throat tightness, hoarseness
-
● rapid heartbeat
-
● faint
-
● yellowing of the skin or eyes.
-
● Stop taking ciprofloxacin hydrochloride tablets and tell your healthcare provider right away if you get yellowing of your skin or white part of your eyes, or if you have dark urine. These can be signs of a serious reaction to ciprofloxacin hydrochloride tablets (a liver problem).
-
● Skin rash
-
● Skin rash may happen in people taking ciprofloxacin hydrochloride tablets, even after only one dose. Stop taking ciprofloxacin hydrochloride tablets at the first sign of a skin rash and call your healthcare provider. Skin rash may be a sign of a more serious reaction to ciprofloxacin hydrochloride tablets.
-
● Serious heart rhythm changes (QT prolongation and torsade de pointes)
-
● Tell your healthcare provider right away if you have a change in your heart beat (a fast or irregular heartbeat), or if you faint. Ciprofloxacin hydrochloride tablets may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this event are higher in people:
-
● who are elderly
-
● with a family history of prolonged QT interval
-
● with low blood potassium (hypokalemia)
-
● who take certain medicines to control heart rhythm (antiarrhythmics)
-
● Intestine infection (Pseudomembranous colitis)
-
● Pseudomembranous colitis can happen with most antibiotics, including ciprofloxacin hydrochloride tablets. Call your healthcare provider right away if you get watery diarrhea, diarrhea that does not go away, or bloody stools. You may have stomach cramps and a fever. Pseudomembranous colitis can happen 2 or more months after you have finished your antibiotic.
-
● Changes in sensation and possible nerve damage (Peripheral Neuropathy)
-
● Damage to the nerves in arms, hands, legs, or feet can happen in people who take fluoroquinolones, including ciprofloxacin hydrochloride tablets. Talk with your healthcare provider right away if you get any of the following symptoms of peripheral neuropathy in your arms, hands, legs, or feet:
-
● pain
-
● burning
-
● tingling
-
● numbness
-
● weakness
-
● Ciprofloxacin hydrochloride tablets may need to be stopped to prevent permanent nerve damage.
-
● Low blood sugar (hypoglycemia)
-
● Sensitivity to sunlight (photosensitivity)
-
● SeeWhat should I avoid while taking ciprofloxacin hydrochloride tablets?
-
● Joint Problems
-
● Increased chance of problems with joints and tissues around joints in children under 18 years old. Tell your child's healthcare provider if your child has any joint problems during or after treatment with ciprofloxacin hydrochloride tablets.
-
● The most common side effects of ciprofloxacin hydrochloride tablets include:
-
● nausea
-
● diarrhea
-
● vomiting
-
● vaginal yeast infection
-
● changes in liver function tests pain or discomfort in the abdomen
-
● These are not all the possible side effects of ciprofloxacin hydrochloride tablets. Tell your healthcare provider about any side effect that bothers you, or that does not go away.
-
● Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
-
● Keep ciprofloxacin hydrochloride tablets and all medicines out of the reach of children.
-
● General Information about ciprofloxacin hydrochloride tablets
-
● Inactive ingredients: cornstarch, microcrystalline cellulose, silicon dioxide, crospovidone, magnesium stearate, polyvinyl alcohol, talc, titanium dioxide, polyethylene glycol and purified water
-
● This Medication Guide has been approved by the U.S. Food and Drug Administration.
-
● Manufactured for:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
INACTIVE INGREDIENT
STARCH, CORNCELLULOSE, MICROCRYSTALLINE
silicon dioxide
crospovidone
magnesium stearate
polyvinyl alcohol
talc
titanium dioxide
polyethylene glycol
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


CiprofloxacinCiprofloxacin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!