Hydrocodone Bitartrate and Acetaminophen
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
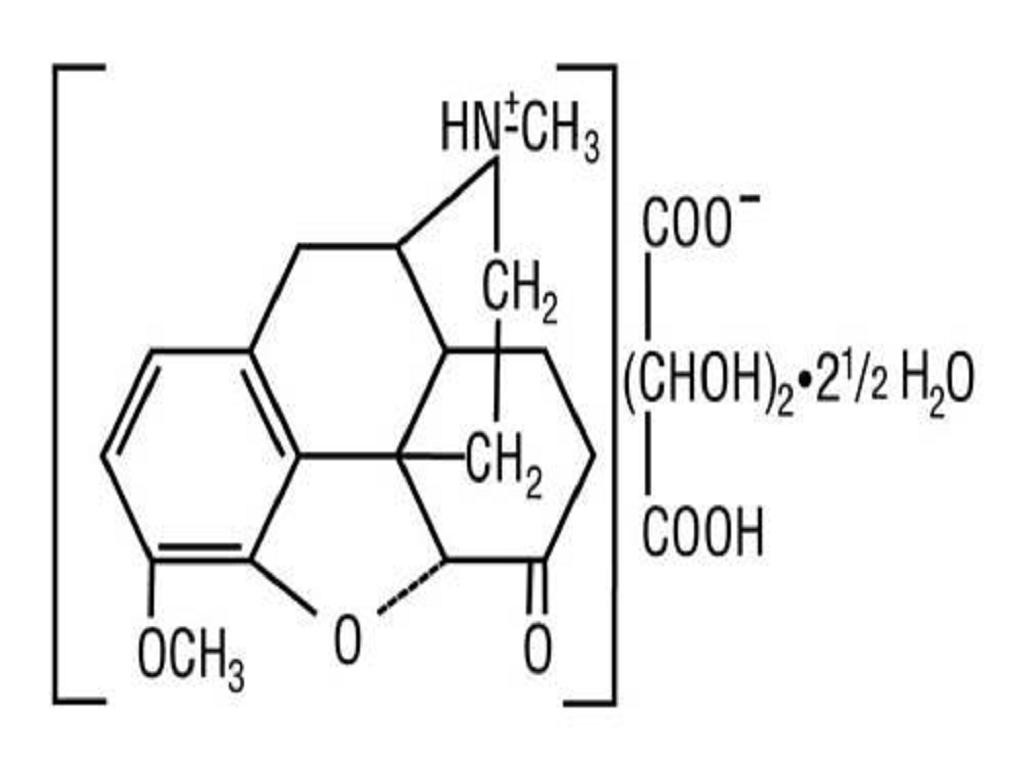
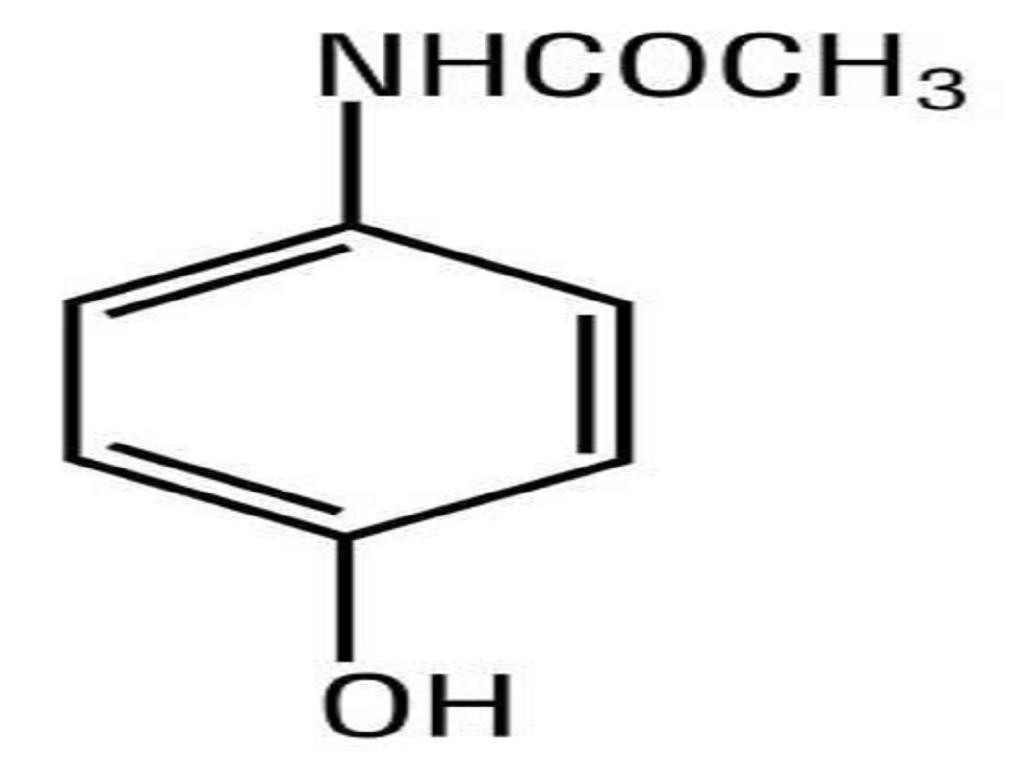
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
OVERDOSAGE
OVERDOSAGE
INDICATIONS & USAGE
INDICATIONS AND USAGEHYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
CONTRAINDICATIONSWARNINGS
WARNINGSRespiratory Depression
Head Injury and Increased Intracranial Pressure
Acute Abdominal Conditions
Misuse, Abuse, and Diversion of Opioids
DRUG ABUSE AND DEPENDENCE
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
LABORATORY TESTS
Laboratory TestsDRUG INTERACTIONS
Drug InteractionsDRUG & OR LABORATORY TEST INTERACTIONS
Drug/Laboratory Test InteractionsCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityLABOR & DELIVERY
Labor and DeliveryNURSING MOTHERS
Nursing MothersPEDIATRIC USE
Pediatric UseGERIATRIC USE
Geriatric UseHYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
ADVERSE REACTIONSOVERDOSAGE
DRUG ABUSE AND DEPENDENCE
DRUG ABUSE AND DEPENDENCEMisuse, Abuse, and Diversion of Opioids
OVERDOSAGE
OVERDOSAGESigns and Symptoms
DOSAGE & ADMINISTRATION
STORAGE AND HANDLING
INACTIVE INGREDIENT
SILICON DIOXIDEMAGNESIUM STEARATE
CELLULOSE, MICROCRYSTALLINE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Hydrocodone Bitartrate and AcetaminophenHydrocodone Bitartrate and Acetaminophen TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!