Isoniazid
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- ISONIAZID DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ISONIAZID CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- ISONIAZID ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ISONIAZID DESCRIPTION
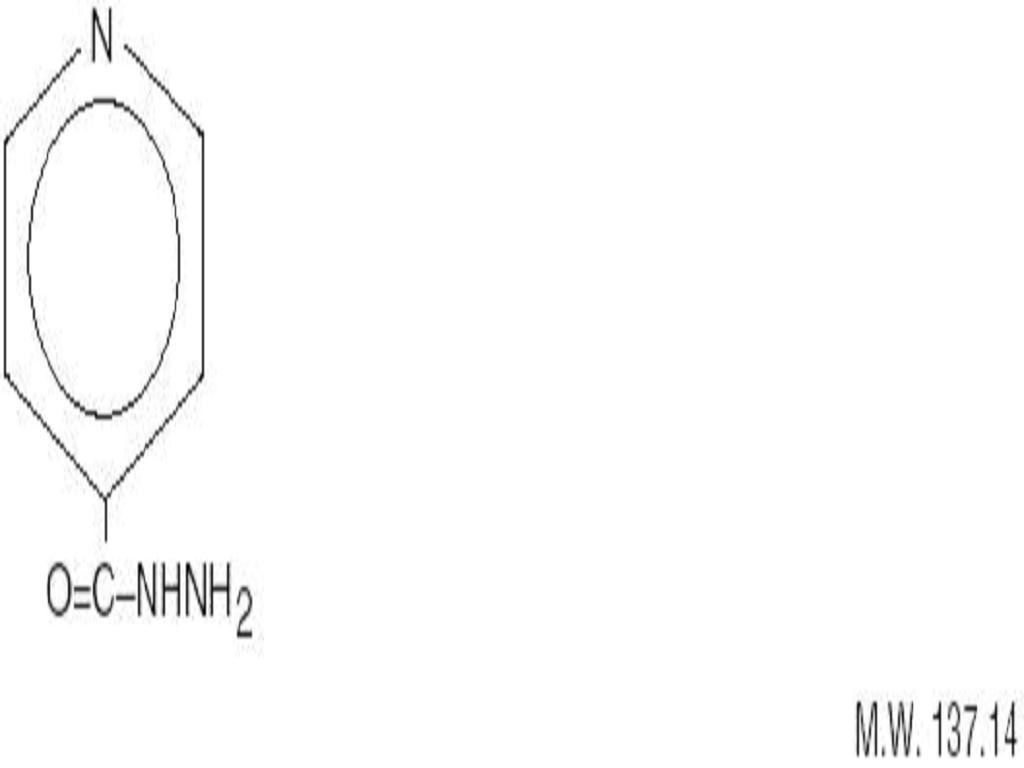
CLINICAL PHARMACOLOGY
Mechanism of Action
Microbiology
INDICATIONS & USAGE
ISONIAZID CONTRAINDICATIONS
WARNINGS
WARNINGPRECAUTIONS
GeneralLABORATORY TESTS
DRUG INTERACTIONS
Acetaminophen
Carbamazepine
Ketoconazole
Phenytoin
Theophylline
Valproate
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic effects: Pregnancy Category CNonteratogenic effects
NURSING MOTHERS
ISONIAZID ADVERSE REACTIONS
WARNING
Gastrointestinal Reactions - Nausea, vomiting, and epigastric distress.
Hypersensitivity Reactions - Fever, skin eruptions (morbilliform, maculopapular, purpuric, or exfoliative), lymphadenopathy, and vasculitis.
Metabolic and Endocrine Reactions - Pyridoxine deficiency, pellagra, hyperglycemia, metabolic acidosis, and gynecomastia.
Miscellaneous Reactions - Rheumatic syndrome and systemic lupus erythematosus-like syndrome.
To report SUSPECTED ADVERSE REACTIONS, contact West-ward Pharmaceutical Corp. at 1-877-233-2001, and the FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch
OVERDOSAGE
Signs and SymptomsTreatment
For the Asymptomatic Patient
For the Symptomatic Patient
General
Rapid control of metabolic acidosis
Dialysis
DOSAGE & ADMINISTRATION
INDICATIONS-
●
-
● Patients with Pulmonary Tuberculosis Without HIV Infection
Patients with Pulmonary Tuberculosis and HIV Infection
Patients with Extra pulmonary Tuberculosis
Pregnant Women with Tuberculosis
Treatment of Patients with Multi-Drug Resistant Tuberculosis (MDRTB)
Directly Observed Therapy (DOT)
For Preventative Therapy of Tuberculosis
HOW SUPPLIED
-
● Bottles of 100 tablets
-
● Bottles of 1000 tablets
-
● Unit Dose Boxes of 100 tablets
-
● Isoniazid Tablets USP, 300 mg: White, round, scored tablets imprinted "West-ward 261".
-
● Bottles of 100 tablets
-
● Unit Dose Boxes of 100 tablets
-
●
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

IsoniazidIsoniazid TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!