Lisinopril and Hydrochlorthiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LISINOPRIL AND HYDROCHLORTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LISINOPRIL AND HYDROCHLORTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LISINOPRIL AND HYDROCHLORTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
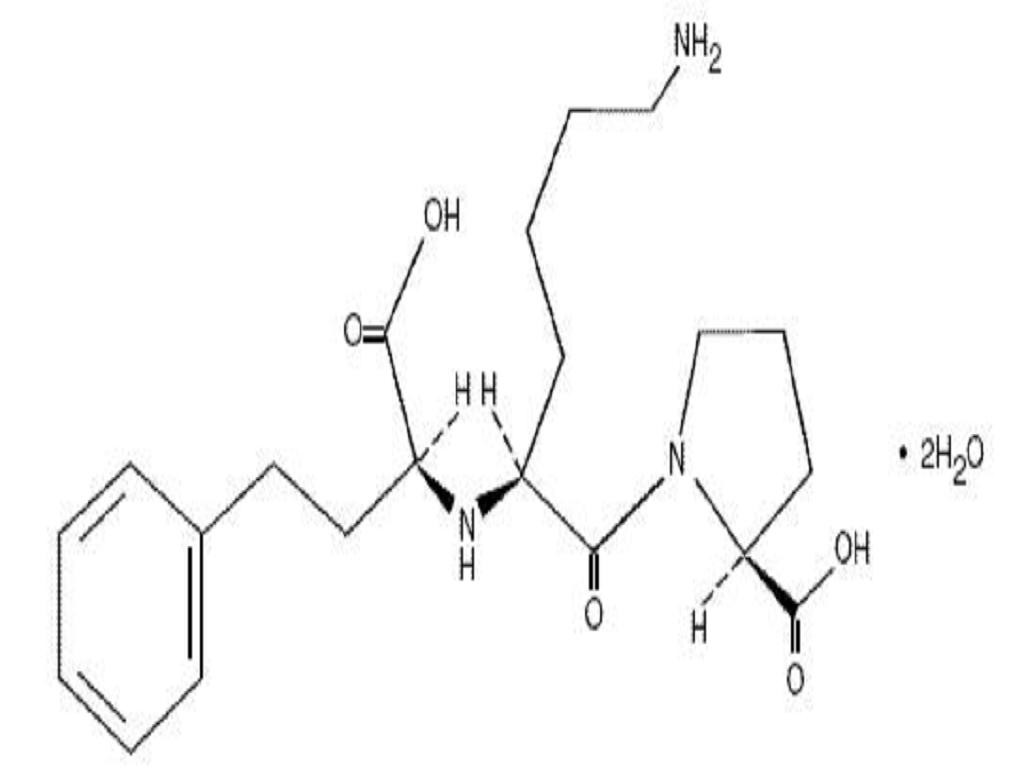
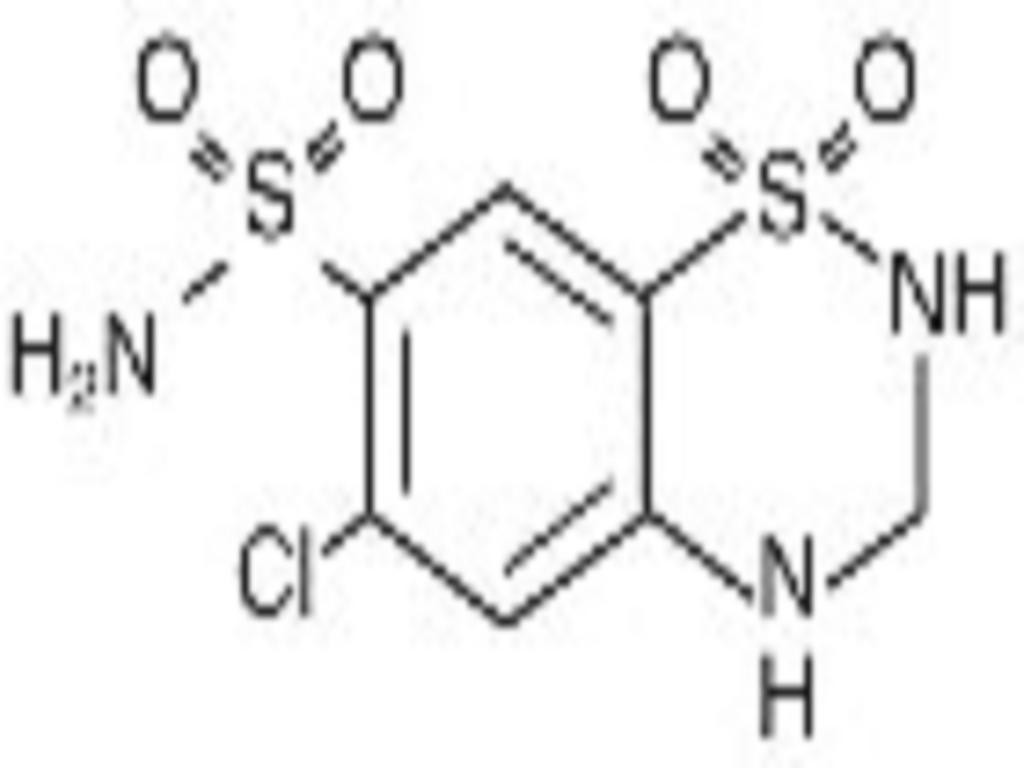
LISINOPRIL AND HYDROCHLORTHIAZIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Lisinopril and HydrochlorothiazideLisinopril
Mechanism of Action
Pharmacokinetics and Metabolism
Pharmacodynamics
Hydrochlorothiazide
INDICATIONS & USAGE
LISINOPRIL AND HYDROCHLORTHIAZIDE CONTRAINDICATIONS
WARNINGS
GeneralLisinopril
Hydrochlorothiazide
Pregnancy
Lisinopril and Hydrochlorothiazide
Lisinopril
Hydrochlorothiazide
PRECAUTIONS
GeneralLisinopril
Hydrochlorothiazide
Information for Patients
Drug Interactions
Lisinopril
Hydrochlorothiazide
Carcinogenesis, Mutagenesis, Impairment of Fertility
Lisinopril and Hydrochlorothiazide
Lisinopril
Hydrochlorothiazide
Pregnancy
Nursing Mothers
Pediatric Use
Geriatric Use
LISINOPRIL AND HYDROCHLORTHIAZIDE ADVERSE REACTIONS
Clinical Laboratory Test Findings
Lisinopril
Hydrochlorothiazide
OVERDOSAGE
DOSAGE & ADMINISTRATION
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Lisinopril and HydrochlorthiazideLisinopril and Hydrochlorthiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!