Meloxicam
FULL PRESCRIBING INFORMATION: CONTENTS*
- SPL INDEXING DATA ELEMENTS
- BOXED WARNING
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- DOSAGE FORMS & STRENGTHS
- MELOXICAM CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- MELOXICAM ADVERSE REACTIONS
- DRUG INTERACTIONS
- USE IN SPECIFIC POPULATIONS
- OVERDOSAGE
- MELOXICAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- NONCLINICAL TOXICOLOGY
- CLINICAL STUDIES
- HOW SUPPLIED
- INFORMATION FOR PATIENTS
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
SPL INDEXING DATA ELEMENTS
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use meloxicam safely and effectively. See full prescribing information for meloxicam tablets.Meloxicam Tablets, USP
Initial U.S. Approval: 2000
WARNING: CARDIOVASCULAR and GASTROINTESTINAL RISKS
-
● NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk.(5.1)
-
● Meloxicam is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery.(4.2,5.1)
-
●
(5.2)
-
● INDICATIONS AND USAGE
(1.1)
-
● Rheumatoid Arthritis (RA)(1.2)
-
● DOSAGE AND ADMINISTRATION
(2.2)(2.3)
-
● Starting dose: 7.5 mg once daily
-
● Dose may be increased to 15 mg once daily
-
● DOSAGE FORMS AND STRENGTHS
-
● CONTRAINDICATIONS
-
● History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs(4.1)
-
● Use during the peri-operative period in the setting of coronary artery bypass graft (CABG) surgery(4.2)
-
● WARNINGS AND PRECAUTIONS
-
● Serious gastrointestinal (GI) adverse events which can be fatal. The risk is greater in patients with a prior history of ulcer disease or GI bleeding, and in patients at higher risk for GI events, especially the elderly.(5.2)
-
● Elevated liver enzymes, and rarely, severe hepatic reactions. Discontinue use immediately if abnormal liver enzymes persist or worsen.(5.3)
-
● New onset or worsening of hypertension. Blood pressure should be monitored closely during treatment.(5.4)
-
● Fluid retention and edema. Should be used with caution in patients with fluid retention or heart failure.(5.5)
-
● Renal papillary necrosis and other renal injury with long-term use. Use with caution in the elderly, those with impaired renal function, heart failure, liver dysfunction, and those taking diuretics, ACE-inhibitors, or angiotensin II antagonists. The use of meloxicam in patients with severe renal impairment is not recommended.(5.6)
-
● Serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal and can occur without warning. Discontinue meloxicam at first appearance of rash or skin reactions.(5.8)
-
● ADVERSE REACTIONS
-
● Adverse events observed in pediatric studies were similar in nature to the adult clinical trial experience(6.1)
-
● To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
(7.7)
-
● Concomitant use of meloxicam and aspirin is not generally recommended because of the potential of increased adverse effect including increased GI bleeding(7.2)
-
● Concomitant use with meloxicam increases lithium plasma levels(7.4)
-
● Concomitant use with NSAIDs may reduce the antihypertensive effect of ACE-inhibitors(7.1)
-
● USE IN SPECIFIC POPULATIONS
-
● Nursing Mothers: Use with caution, as meloxicam may be excreted in human milk(8.3)
-
●
See17for PATIENT COUNSELING INFORMATION and the FDA-approved Medication Guide
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF SERIOUS CARDIOVASCULAR and GASTROINTESTINAL EVENTS
1 INDICATIONS AND USAGE
1.1 Osteoarthritis (OA)
1.2 Rheumatoid Arthritis (RA)
2 DOSAGE AND ADMINISTRATION
2.1 General Instructions
2.2 Osteoarthritis
2.3 Rheumatoid Arthritis
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Allergic Reactions
4.2 Coronary Surgery
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Thrombotic Events
5.2 Gastrointestinal (GI) EffectsRisk of GI Ulceration, Bleeding, and Perforation
5.3 Hepatic Effects
5.4 Hypertension
5.5 Congestive Heart Failure and Edema
5.6 Renal Effects
5.7 Anaphylactoid Reactions
5.8 Adverse Skin Reactions
5.9 Pregnancy
5.10 Corticosteroid Treatment
5.11 Masking of Inflammation and Fever
5.12 Hematological Effects
5.13 Use in Patients with Pre-existing Asthma
5.14 Monitoring
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 ACE-inhibitors
7.2 Aspirin
7.3 Diuretics
7.4 Lithium
7.5 Methotrexate
7.6 Cyclosporine
7.7 Warfarin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Osteoarthritis and Rheumatoid Arthritis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Medication Guide
17.2 Cardiovascular Effects
17.3 Gastrointestinal Effects
17.4 Hepatotoxicity
17.5 Adverse Skin Reactions
17.6 Weight Gain and Edema
17.7 Anaphylactoid Reactions
17.8 Effects During Pregnancy
MEDICATION GUIDE
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 7.5 mg (100 Tablet Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 7.5 mg Bulk Tablet Label
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 15 mg (100 Tablet Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 15 mg Bulk Tablet Label
*Sections or subsections omitted from the full prescribing information are not listed
BOXED WARNING
WARNING: RISK OF SERIOUS CARDIOVASCULAR and GASTROINTESTINAL EVENTS
-
● Nonsteroidal anti-inflammatory drugs (NSAIDs) may cause an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk [seeWarnings and Precautions (5.1)].
-
● Meloxicam is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery [seeContraindications (4.2)andWarnings and Precautions (5.1)].
-
● Gastrointestinal Risk
-
●
INDICATIONS & USAGE
1 INDICATIONS AND USAGE1.1 Osteoarthritis (OA)
Clinical Studies (14.1)
1.2 Rheumatoid Arthritis (RA)
Clinical Studies (14.1)
DOSAGE & ADMINISTRATION
2 DOSAGE AND ADMINISTRATION2.1 General Instructions
Warnings and Precautions (5.4)
Warnings and Precautions (5.6)Use in Specific Populations (8.7)Clinical Pharmacology (12.3)
2.2 Osteoarthritis
2.3 Rheumatoid Arthritis
DOSAGE FORMS & STRENGTHS
3 DOSAGE FORMS AND STRENGTHS-
● 7.5 mg: pastel yellow, round, biconvex, uncoated tablet debossed withC'on one side and79'on the other side.
-
● 15 mg: pastel yellow, oblong, biconvex, uncoated tablet debossed withC'on one side and80'on the other side.
-
●
MELOXICAM CONTRAINDICATIONS
4 CONTRAINDICATIONS4.1 Allergic Reactions
Warnings and Precautions (5.75.13)
4.2 Coronary Surgery
Warnings and Precautions (5.1)
WARNINGS AND PRECAUTIONS
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Thrombotic Events
Contraindications (4.2)
Warnings and Precautions (5.2)
5.2 Gastrointestinal (GI) EffectsRisk of GI Ulceration, Bleeding, and Perforation
5.3 Hepatic Effects
Adverse Reactions (6.1)
Use in Specific Populations (8.6)Clinical Pharmacology (12.3)
5.4 Hypertension
5.5 Congestive Heart Failure and Edema
5.6 Renal Effects
Dosage and Administration (2.1)Use in Specific Populations (8.7)Clinical Pharmacology (12.3)
5.7 Anaphylactoid Reactions
Contraindications (4.1)Warnings and Precautions (5.12)
5.8 Adverse Skin Reactions
5.9 Pregnancy
Use in Specific Populations (8.1)Patient Counseling Information (17.8)
5.10 Corticosteroid Treatment
5.11 Masking of Inflammation and Fever
5.12 Hematological Effects
5.13 Use in Patients with Pre-existing Asthma
5.14 Monitoring
MELOXICAM ADVERSE REACTIONS
6 ADVERSE REACTIONS-
● Cardiovascular thrombotic events [seeBoxed WarningandWarnings and Precautions (5.1)]
-
● Gastrointestinal effectsrisk of GI ulceration, bleeding, and perforation [seeBoxed WarningandWarnings and Precautions (5.2)]
-
● Hepatic effects [seeWarnings and Precautions (5.3)]
-
● Hypertension [seeWarnings and Precautions (5.4)]
-
● Congestive heart failure and edema [seeWarnings and Precautions (5.5)]
-
● Renal effects [seeWarnings and Precautions (5.6)]
-
● Anaphylactoid reactions [seeWarnings and Precautions (5.7)]
-
● Adverse skin reactions [seeWarnings and Precautions (5.8)]
-
●
Adults
PlaceboMeloxicamMeloxicamDiclofenac7.5 mg daily15 mg daily100 mg dailyNo. of Patients157154156153GastrointestinalBody as a WholeCentral and Peripheral Nervous SystemRespiratorySkin
PlaceboMeloxicamMeloxicam7.5mg daily15mg dailyNo. of Patients469481477Gastrointestinal DisordersGeneral Disorders and Administration Site ConditionsInfection and InfestationsMusculoskeletal and Connective Tissue DisordersNervous System DisordersSkin and Subcutaneous Tissue Disorders
4 to 6 Weeks Controlled Trials6 Month Controlled TrialsMeloxicamMeloxicamMeloxicamMeloxicam7.5 mg daily15 mg daily7.5 mg daily15 mg dailyNo. of Patients8955256169306GastrointestinalBody as a WholeCentral and Peripheral Nervous SystemHematologicMusculoskeletalPsychiatricSkinUrinary
Body as a WholeCardiovascularCentral andPeripheralNervous SystemGastrointestinalHeart Rate andRhythmHematologicLiver and BiliarySystemMetabolic andNutritionalPsychiatricRespiratorySkin andAppendagesSpecial SensesUrinary System
6.2 Postmarketing Experience
DRUG INTERACTIONS
7 DRUG INTERACTIONSClinical Pharmacology (12.3)
7.1 ACE-inhibitors
7.2 Aspirin
7.3 Diuretics
Warnings and Precautions (5.6)
7.4 Lithium
7.5 Methotrexate
Clinical Pharmacology (12.3)
7.6 Cyclosporine
7.7 Warfarin
Clinical Pharmacology (12.3)
USE IN SPECIFIC POPULATIONS
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy
Warnings and Precautions (5.9)Patient Counseling Information (17.8)
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
Warnings and Precautions (5.3) Clinical Pharmacology (12.3)
8.7 Renal Impairment
Dosage and Administration (2.1) Warnings and Precautions (5.6)Clinical Pharmacology (12.3)
OVERDOSAGE
10 OVERDOSAGEMELOXICAM DESCRIPTION
11 DESCRIPTION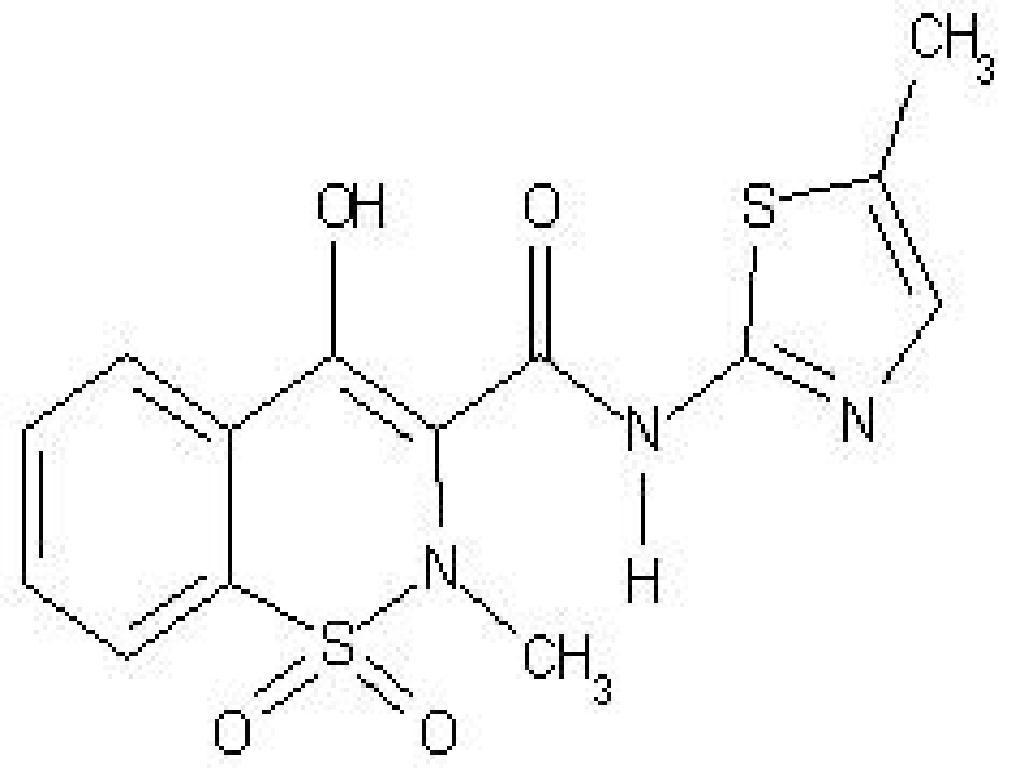
CLINICAL PHARMACOLOGY
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
N18581212
Warnings and Precautions (5.3)Use in Specific Populations (8.6)
Warnings and Precautions (5.6)Use in Specific Populations (8.7)
Dosage and Administration (2.1)Warnings and Precautions (5.6)Use in Specific Populations (8.7)
Drug Interactions (7.2)
Drug Interactions (7.4)
Drug Interactions (7.5)
Drug Interactions (7.7)
NONCLINICAL TOXICOLOGY
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
CLINICAL STUDIES
14 CLINICAL STUDIES14.1 Osteoarthritis and Rheumatoid Arthritis
HOW SUPPLIED
16 HOW SUPPLIED/STORAGE AND HANDLINGINFORMATION FOR PATIENTS
17 PATIENT COUNSELING INFORMATIONFDA-approved Medication Guide
17.1 Medication Guide
17.2 Cardiovascular Effects
Warnings and Precautions (5.1)
17.3 Gastrointestinal Effects
Warnings and Precautions (5.2
17.4 Hepatotoxicity
Warnings and Precautions (5.3)
17.5 Adverse Skin Reactions
Warnings and Precautions (5.8)
17.6 Weight Gain and Edema
Warnings and Precautions (5.5)
17.7 Anaphylactoid Reactions
Warnings and Precautions (5.7)
17.8 Effects During Pregnancy
Warnings and Precautions (5.9)Use in Specific Populations (8.1)
SPL MEDGUIDE
MEDICATION GUIDE-
● with longer use of NSAID medicines
-
● in people who have heart disease
-
●
-
● may cause death
-
●
-
● longer use
-
● smoking
-
● drinking alcohol
-
● older age
-
● having poor health
-
●
-
● at the lowest dose possible for your treatment
-
● for the shortest time needed
-
●
-
● menstrual cramps and other types of short-term pain
-
●
-
● for pain right before or after heart bypass surgery
-
●
-
● about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist.
-
● if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
-
● if you are breastfeeding. Talk to your doctor.
-
●
-
● stroke
-
● high blood pressure
-
● heart failure from body swelling (fluid retention)
-
● kidney problems including kidney failure
-
● bleeding and ulcers in the stomach and intestine
-
● low red blood cells (anemia)
-
● life-threatening skin reactions
-
● life-threatening allergic reactions
-
● liver problems including liver failure
-
● asthma attacks in people who have asthma
-
●
-
● constipation
-
● diarrhea
-
● gas
-
● heartburn
-
● nausea
-
● vomiting
-
● dizziness
-
●
-
● chest pain
-
● weakness in one part or side of your body
-
● slurred speech
-
● swelling of the face or throat
-
●
-
● more tired or weaker than usual
-
● itching
-
● your skin or eyes look yellow
-
● stomach pain
-
● flu-like symptoms
-
● vomit blood
-
● there is blood in your bowel movement or it is black and sticky like tar
-
● skin rash or blisters with fever
-
● unusual weight gain
-
● swelling of the arms and legs, hands and feet
-
●
Generic NameTradename
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MeloxicamMeloxicam TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!