Morphine Sulfate
International Medication Systems, Limited
MORPHINE SULFATE INJECTION, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- MORPHINE SULFATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- MORPHINE SULFATE INDICATIONS AND USAGE
- MORPHINE SULFATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- MORPHINE SULFATE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- MORPHINE SULFATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Carton - 30mg/30mL (Stock No. 1911)
- Syringe Label - 30mg/30mL (Stock No. 1911)
FULL PRESCRIBING INFORMATION
DESCRIPTION
Morphine is a tertiary nitrogen base containing a phenanthrene nucleus; it has two hydroxyl groups, one phenolic and the other alcoholic (secondary). The sulfate salt occurs as white, feathery, silky crystals, cubical masses of crystals, or white, crystalline powder.
The chemical name of morphine sulfate is 7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol sulfate (2:1) (salt), pentahydrate.
The molecular formula is (C17H19NO3)2 • H2SO4 • 5H2O, and the structural formula is as follows:

Morphine Sulfate Injection USP, is a sterile solution of morphine sulfate pentahydrate in Water for Injection, USP. Disodium edetate (0.075%) and sodium bisulfite (0.1%) have been added as stabilizer and antioxidant, respectively. It is available in the following concentration of morphine sulfate per mL: 1 mg. (See HOW SUPPLIED).
This product contains no bacteriostat or antimicrobial agents and is intended as a single dose unit. When the dosing requirement is completed, the unused portion should be discarded in an appropriate manner.
CLINICAL PHARMACOLOGY
Morphine is the principal narcotic alkaloid of opium, from which it differs somewhat in its mode of action. Its effects are remarkably diverse and include analgesia, drowsiness, changes in mood, mental clouding, respiratory depression, decreased gastrointestinal motility, nausea, vomiting, and alterations of the endocrine and autonomic nervous systems. Its most important actions are on the brain, especially its higher functions. An initial transitory stimulation is followed by depression of the brain, its higher functions and medullary centers. Other actions include depression of the cough center, release of antidiuretic hormone, pupillary constriction, increase in biliary tract pressure, sedation with muscle relaxation, decreased physical activity, lassitude, increased tone of the gastrointestinal and genitourinary tracts, mild vasodilation, and an increased amplitude of ureteral contractions.
Opioids act as agonists, interacting with stereospecific and saturable binding sites or receptors in the brain and other tissues.
Morphine is about two-thirds absorbed from the gastrointestinal tract with the maximum analgesic effect occurring 60 minutes post administration. Onset of analgesia following intramuscular or subcutaneous administration occurs within 10 to 30 minutes. The effect persists for 4 to 5 hours.
Approximately 90% of a parenteral dose of morphine appears in the urine within 24 hours as the product of glucuronide conjugation. Most of the remainder is excreted in the bile and eliminated in the feces.
MORPHINE SULFATE INDICATIONS AND USAGE
Morphine sulfate is indicated for the relief of severe pain. It is used preoperatively to sedate the patient and allay apprehension, facilitate anesthesia induction and reduce anesthetic dosage. It is likewise effective in the control of postoperative pain.
The use of morphine for the relief of pain should be reserved for the more severe manifestations of pain, as in myocardial infarction,severe injuries, or in severe chronic pain associated with terminal cancer after all non-narcotic analgesics have failed.
Effective analgesic therapy of severe chronic pain associated with terminal cancer continues to be a difficult problem. Intermittent administration of intramuscular morphine may be effective; however, the mode of therapy has significant limitations. Morphine has a short plasma half-life of 2.5 to 3.0 hours; therefore, frequent administration (every 1 to 2 hours) often becomes necessary to control severe pain associated with cancer. Tolerance develops to the analgesic effects and increasingly higher doses of morphine are required to produce analgesia. The higher morphine doses produce significant and often life-threatening side effects (see “ADVERSE REACTIONS”). The peak and trough effects produced by intermittent administration cause fluctuations in pain control. Repeated I.M. injections are frequently unacceptable due to the lack of muscle mass in the debilitated patient, the tendency for bruising and bleeding at the injection site, and the anxiety and pain associated with the injection.
Continuous I.V. infusion of morphine (see “DOSAGE AND ADMINISTRATION”) has been employed as an alternative to the traditional modes of administration. Lower doses of morphine produce uniform pain control because a steady morphine concentration is maintained. Titration of the dosage to the patient’s needs is easily achieved by adjusting the infusion rate. The lag time between the patient’s request for pain medication and administration of the dose and the amount of nursing time necessary for preparation and administration of frequent doses are reduced. The degree of respiratory depression and sedation may be decreased, and the anxiety experienced by the patient in anticipation of I.M. administration is avoided. Some investigators feel that tolerance to the analgesic effects may develop more slowly with continuous I.V. infusion.
In addition to analgesia, the drug may relieve anxiety and reduce left ventricular work by reducing preload pressure. Morphine is also used in the therapy of dyspnea associated with acute left ventricular failure and pulmonary edema. Care must be taken to avoid inducing respiratory depression in such patients.
For open-heart surgery, especially in high risk patients with cardiac disease, some anesthesiologists use morphine to produce anesthesia.
MORPHINE SULFATE CONTRAINDICATIONS
Hypersensitivity (allergy) to morphine sulfate is one of the contraindications to its use. Because of its stimulating effect on the spinal cord, morphine should not be used in convulsive states, such as those occurring in status epilepticus, tetanus, and strychnine poisoning. Morphine is also contraindicated in the following conditions: respiratory insufficiency or depression; bronchial asthma; heart failure secondary to chronic lung disease; cardiac arrhythmias; increased intracranial or cerebrospinal pressure; head injuries; brain tumor; acute alcoholism; and delirium tremens.
WARNINGS
Contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Morphine is a potent medicinal agent of great utility but also with considerable potential for harmful effect, including psychological and physical tolerance and dependence. Withdrawal will occur on abrupt discontinuation or administration of a narcotic antagonist. Morphine and other opiates produce relaxation, indifference to pain and stress, lethargy, and euphoria. Patients who receive narcotics regularly for more than a few days may exhibit mild symptoms upon discontinuation of therapy, which may not be recognized as withdrawal. However, the majority of patients who receive opiates for medical reasons do not develop drug seeking behavior or compulsive drug use. Personality characteristics play a major role in determining which patients are likely to abuse drugs. Morphine should be used only if other drugs with fewer hazards are inadequate, and with the recognition of the possibility that it may mask significant manifestations of disease which should be identified for proper diagnosis and treatment.
PRECAUTIONS
GENERAL:
Parenteral Therapy – Give by very slow I.V. injection, preferably in the form of a diluted solution. Rapid I.V. injection of morphine and other narcotic analgesics increases the incidence of adverse reactions; severe respiratory depression, hypotension, apnea, peripheral circulatory collapse, cardiac arrest, and anaphylactic reactions have occurred. These preparations should not be administered I.V. unless a narcotic antagonist and facilities for assisted or controlled respiration are immediately available. When given parenterally, especially I.V., the patient should be lying down. Use caution when injecting subcutaneously or intramuscularly in chilled areas or in patients with hypotension or shock, since impaired perfusion may prevent complete absorption. If repeated injections are administered, an excessive amount may be suddenly absorbed if normal circulation is reestablished.
Head Injury and Increased Intracranial Pressure – The respiratory depressant effects of morphine and its capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, brain tumor, other intracranial lesions, or a preexisting increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Asthma and Other Respiratory Conditions – The use of bisulfites is contraindicated in asthmatics. Bisulfites and morphine may potentiate each other, preventing use by causing severe adverse reactions. Use with extreme caution in patients having an acute asthmatic attack, patients with chronic obstructive pulmonary disease or cor pulmonale, patients with a substantially decreased respiratory reserve, and patients with preexisting respiratory depression, hypoxia or hypercapnia. In such patients, even usual therapeutic doses of narcotics may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea.
Hypotensive Effect – Administration may result in severe hypotension in the postoperative patient or in any individual whose ability to maintain blood pressure has already been compromised by a depleted blood volume or concurrent administration of drugs such as phenothiazines or general anesthetics. Narcotics may produce orthostatic hypotension in ambulatory patients.
Supraventricular Tachycardias – Caution should be used in patients with atrial flutter and other supraventricular tachycardias due to a possible vagolytic action which may produce a significant increase in the ventricular response rate.
Acute Abdominal Conditions – The administration of morphine or other narcotics may obscure the diagnosis or clinical course in patients with acute abdominal conditions. Use also with caution in patients with gastrointestinal hemorrhage, ulcerative colitis, or recent GI or urinary tract surgery.
Special-Risk Patients – Caution must be exercised in elderly and debilitated patients and in patients who are known to be sensitive to CNS depressants, including those with cardiovascular or pulmonary disease, myxedema, acute alcoholism, delirium tremens, cerebral arteriosclerosis, emphysema, fever, bronchial asthma, kyphoscoliosis, Addison’s disease, prostatic hypertrophy or urethral stricture, toxic psychosis.
Renal and Hepatic Dysfunction – Morphine may have a prolonged duration and cumulative effect in patients with renal or hepatic dysfunction.
Convulsions – Morphine may aggravate preexisting convulsive disorders. Convulsions may occur in individuals without a history of convulsive disorders if dosage is substantially escalated above recommended levels because of tolerance development.
PATIENTS INFORMATION:
Morphine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery.
Use with caution and in reduced doses in patients currently receiving other narcotic analgesics, general anesthetics, antihistamines; phenothiazines, barbiturates, other tranquilizers, sedative-hypnotics, tricyclic antidepressants and other CNS depressants (including alcohol). Respiratory depression, hypotension, profound sedation, or coma may result.
The analgesic effect of morphine is potentiated by chlorpromazine and methocarbamol.
Morphine may increase the anticoagulant activity of coumarin and other anticoagulants. Concurrent administration of cimetidine and morphine has been reported to precipitate apnea, confusion and muscle twitching in an isolated report.
Monoamine oxidase inhibitors markedly potentiate the action of morphine.
Neostigmine increases the intensity and duration of the analgesic action of morphine. When morphine is to be administered to patients receiving propiomazine (Largon), the dose of morphine should be reduced by one-quarter to one-half.
Reserpine inhibits the analgesic action of morphine.
Veratrum alkaloids may have additive effects, particularly on respiratory depression, when administered concurrently withmorphine.
Atropine antagonizes morphine respiratory depression. Levallorphan and nalorphine antagonize morphine actions,principally the respiratory depression.
CARINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY:
Morphine has no known carcinogenic or mutagenic potential. However, no long-term animal studies are available to support this observation.
PREGNANCY:
Teratogenic Effects: Pregnancy Category C-
Animal reproduction studies have not been conducted with morphine sulfate. It is not known whether morphine sulfate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. On the basis of the historical use of morphine sulfate during all stages of pregnancy, there is no known risk of fetal abnormality. Morphine sulfate should be given to a pregnant woman only if clearly needed.
Nonteratogenic Effects:
Narcotic analgesics cross the placental barrier; hence, newborn infants whose mothers have been administered such analgesics during labor should be closely observed for signs of respiratory depression and treated for narcotic overdosage if necessary.
ADVERSE REACTIONS
The major hazards associated with morphine therapy include the following: respiratory depression, apnea, and, to a lesser degree, circulatory depression. Respiratory arrest, shock, and cardiac arrest have occurred.
The most frequent adverse reactions include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not suffering from severe pain. In such individuals, lower doses are advisable.
Other adverse reactions include:
CNS: Euphoria, dysphoria, delirium, weakness, headache, somnolence, drowsiness, miosis, pinpoint pupils, coma, insomnia, agitation, tremor, uncoordinated muscle movements, impairment of mental and physical performance, mental clouding, lethargy, anxiety, fear, psychic dependence, mood changes, transient hallucinations, disorientation, confusion, and visual disturbances have been reported.
GI: Dry mouth, anorexia, constipation, and biliary tract spasm. Patients with chronic ulcerative colitis may experience increased colonic motility; toxic dilatation has been reported in patients with acute ulcerative colitis.
Cardiovascular: Flushing of the face, peripheral circulatory collapse, tachycardia, bradycardia, palpitation, faintness, hypotension, syncope, and phlebitis following I.V. injection.
Genito-urinary: Ureteral spasm and spasm of vesical sphincters, urinary retention or hesitancy, oliguria, antidiuretic effect, reduced libido and/or potency.
Allergic: Pruritus, urticaria, other skin rashes, edema, and (rarely) hemorrhagic urticaria. Wheal and flare over the vein with I.V. injection may occur. Anaphylactoid reactions have been reported following I.V. administration. An isolated case of thrombocytopenia has been reported to be induced by morphine.
DRUG ABUSE AND DEPENDENCE
Controlled Substance: Morphine sulfate is a Schedule II narcotic.
Dependence: Morphine can produce drug dependence and, therefore, has the potential for being abused. Patients receiving therapeutic dosage regimens of 10 mg every 4 hours for 1 to 2 weeks have exhibited mild withdrawal symptoms. Dependence is recognizable by an increased tolerance to the analgesic effect and the appearance of purposive phenomena (complaints, pleas, demands, or manipulative actions) shortly before the time of the next scheduled dose. Withdrawal should be treated in a hospital. Usually, it is necessary only to provide supportive care with administration of a tranquilizer to suppress anxiety. Severe symptoms of withdrawal may require administration of a replacement narcotic.
OVERDOSAGE
Signs and Symptoms: Serious overdosage is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, maximally constricted pupils, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, particularly by the I.V. route, apnea, circulatory collapse, cardiac arrest, and death may occur.
Treatment of Overdose: Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. If depressed respiration is associated with muscular rigidity, an I.V. neuromuscular blocking agent may be required to facilitate assisted or controlled respiration.
The narcotic antagonists - nalorphine, naloxone, and levallorphan - are specific antidotes against respiratory depression resulting from overdosage or unusual sensitivity to narcotics. Thus, an antagonist should be administered, preferably by the I.V. route, simultaneously with efforts at respiratory resuscitation. Since the duration of action of morphine may exceed that of the antagonist, repeated doses of the antagonist may be required to maintain adequate respiration; the patient must be kept under surveillance.
Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. In cases of oral overdose, the stomach should be evacuated by emesis or gastric lavage if treatment can be instituted within 2 hours following ingestion. The patient should be observed closely for a rise in temperature or pulmonary complications that may signal the need for institution of antibiotic therapy.
DOSAGE AND ADMINISTRATION
THIS PRODUCT IS FOR SLOW I.V. USE, NOT FOR INTRATHECAL OR EPIDURAL USE.
For Relief of Pain and as Pre-anesthetic: The usual adult dose is 10 mg every 4 hours, depending on the severity of the condition and the patient’s response. The usual individual dose range is 5 to 15 mg. The usual daily dose range is 12 to 120 mg.
Usual Pediatric Subcutaneous: 100 to 200 μg (0.1 to 0.2 mg) per kg body weight, not to exceed 15 mg per dose.
For Open Heart Surgery: Large doses (0.5 to 3 mg/kg) of morphine are administered intravenously as the sole anesthetic or with a suitable anesthetic agent. The patients are given oxygen and cardiovascular function is not depressed by morphine, as long as adequate ventilation is maintained.
For Severe Chronic Pain Associated with Terminal Cancer: Continuous Intravenous Infusion - Prior to the initiation of the morphine infusion (in concentrations between 0.2 to 1 mg/mL), a loading dose of 15 mg or higher of morphine sulfate may be administeredby I.V. push to alleviate pain.
The infusion dosage range is 0.8 mg/hr to 80 mg/hr, though doses of up to 144 mg/hr have been used. Thus, for the 1 mg/mL solution, the infusion may be run from 0.8 mL/hr to 80 mL/hr, and for the 0.5 mg/mL solution, the infusion may be run from 1.6 mL/hr to 160 mL/hr.
A constant infusion rate must be maintained with an infusion pump in order to assure proper dosage control. Care must be taken to avoid overdosage (respiratory depression) or abrupt cessation of therapy, which may give rise to withdrawal symptoms.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
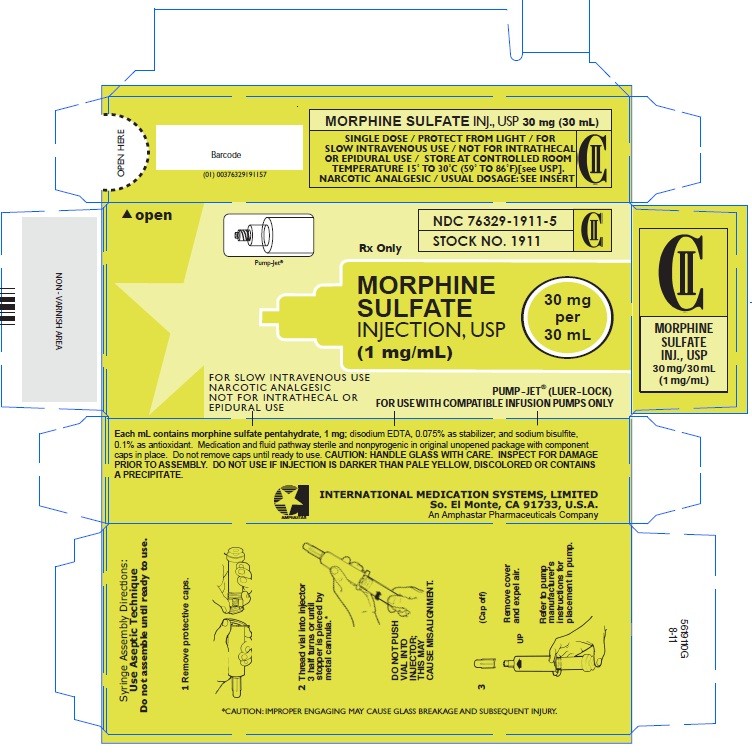
HOW SUPPLIED
In unit-use syringe packages for single dose administration; cartons of 25 unit-use syringe packages:
Concentration System Stock No. NDC No. Route
1 mg/mL 30 mg in 30 mL PUMP-JET® 1911 76329-1911-5 SUITABLE FOR USE WITH COMPATIBLE I.V. INFUSION PUMPS
Syringe Assembly Directions:
USE ASEPTIC TECHNIQUE
Do not assemble until ready to use.
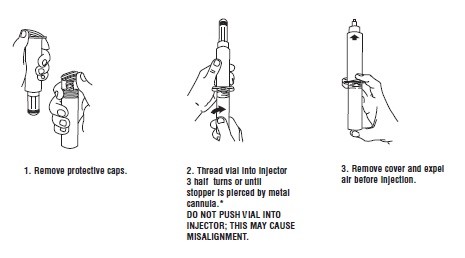
*CAUTION: IMPROPER ENGAGING MAY CAUSE GLASS BREAKAGE AND SUBSEQUENT INJURY.
Store at controlled room temperature 15˚ to 30˚C (59˚ to 86˚F). Morphine sulfate solutions may darken with age. Do not use if injection is darker than pale yellow, discolored in any other way, or contains a precipitate. Protect from light.
NOTE: This product contains antioxidants and is not intended for intrathecal or epidural use.
Rx Only
INTERNATIONAL MEDICATION SYSTEMS, LIMITED
SO. EL MONTE, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company REV. 8-11
Carton - 30mg/30mL (Stock No. 1911)
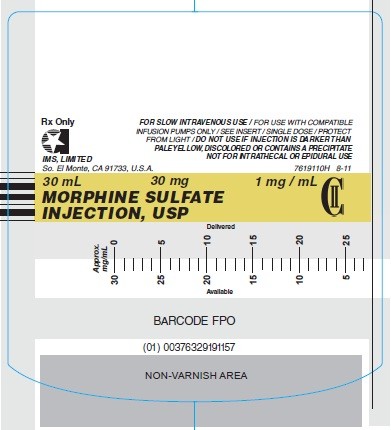
Syringe Label - 30mg/30mL (Stock No. 1911)
Morphine SulfateMorphine Sulfate INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||