Sulfamethoxazole and Trimethoprim
FULL PRESCRIBING INFORMATION: CONTENTS*
- SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
- INACTIVE INGREDIENT
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- MICROBIOLOGY
- INDICATIONS & USAGE
- SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
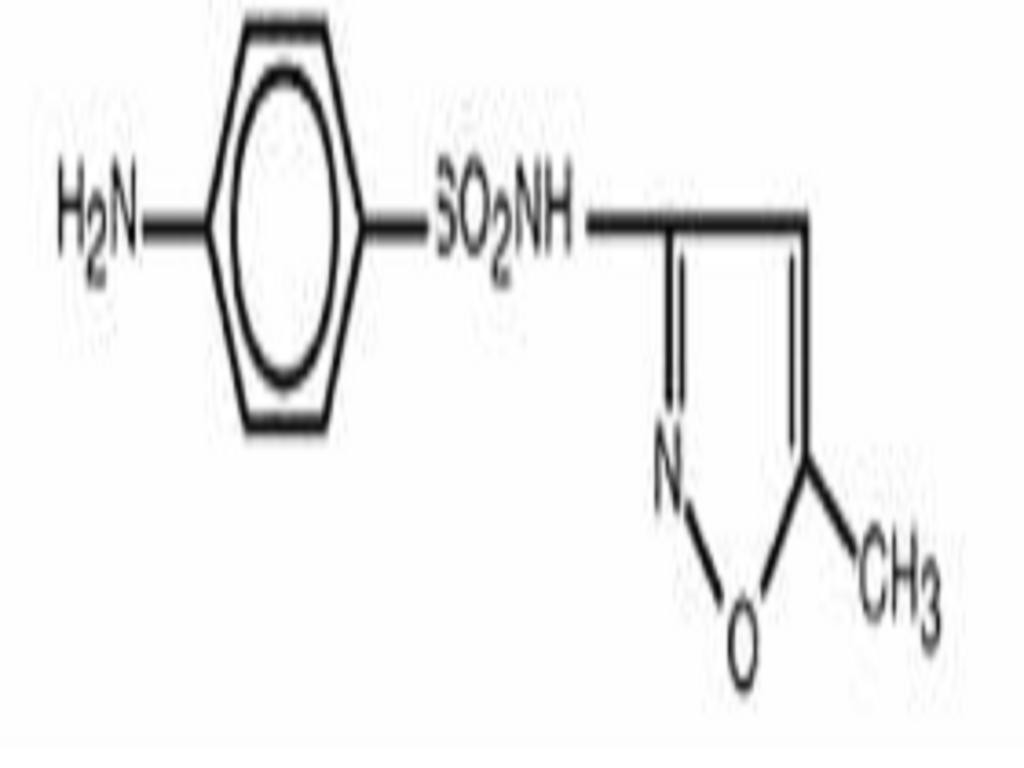
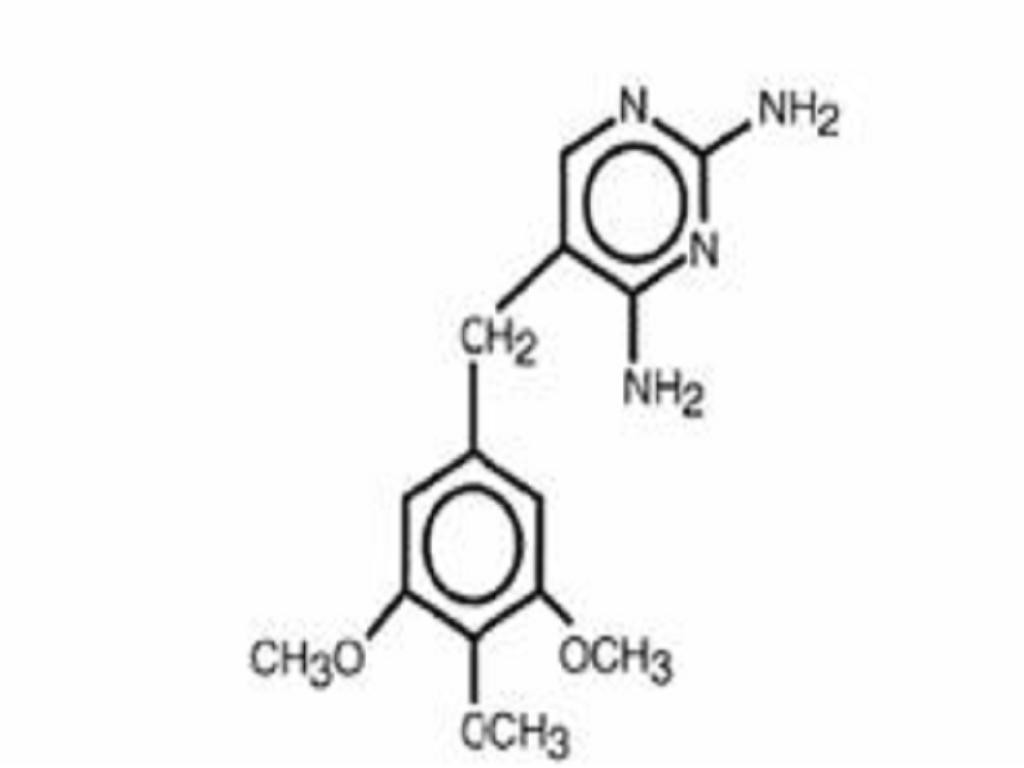
SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
DESCRIPTION
INACTIVE INGREDIENT
Inactive ingredientsCLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
PHARMACOKINETICS
Geriatric PharmacokineticsMICROBIOLOGY
INDICATIONS AND USAGE
Susceptibility Testing Methods
Dilution Techniques
For testing Enterobacteriaceae:MIC (Interpretation
When testing either Haemophilus influenzae*or Streptococcus pneumoniae:MIC (Interpretation*These interpretative standards are applicable only to broth microdilution susceptibility tests with Haemophilus influenzae using Haemophilus Test Medium (HTM)4.These interpretative standards are applicable only to broth microdilution susceptibility tests using cation-adjusted Mueller-Hinton broth with 2% to 5% lysed horse blood4.0.5/9.5Susceptible (S)1/192/38Intermediate (I)4/7Resistant (R)
Quality Control
MicroorganismMIC (*This quality control range is applicable only to Haemophilus influenzae ATCC 49247 tested by broth microdilution procedure using Haemophilus Test Medium (HTM)4.This quality control range is applicable to tests performed by the broth microdilution method only using cation-adjusted Mueller-Hinton broth with 2% to 5% lysed horse blood4.Escherichia coliATCC 259220.5/9.5Haemophilus influenzae*ATCC 492470.03/0.590.25/4.75Streptococcus pneumoniaeATCC 496190.12/2.41/19
Diffusion Techniques
For testing either Enterobacteriaceae or Haemophilus influenzae*:Zone Diameter (mm)Interpretation*These zone diameter standards are applicable only for disk diffusion testing with Haemophilus influenzae and Haemophilus Test Medium (HTM)5.16Susceptible (S)1115Intermediate (I)10Resistant (R)
When testing Streptococcus pneumoniae*:Zone Diameter (mm)Interpretation*These zone diameter interpretative standards are applicable only to tests performed using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood when incubated in 5% CO25.19Susceptible (S)1618Intermediate (I)15Resistant (R)Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for sulfamethoxazole/trimethoprim.
Quality Control
1
MicroorganismZone Diameter Ranges (mm)*This quality control range is applicable only to Haemophilus influenzae ATCC 49247 tested by a disk diffusion procedure using Haemophilus Test Medium (HTM)5.This quality control range is applicable only to tests performed by disk diffusion using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood when incubated in 5% CO25.Escherichia coliATCC 259222432Haemophilus influenzae*ATCC 492472432Streptococcus pneumoniaeATCC 496192028
1
Mueller-Hinton agar should be checked for excessive levels of thymidine or thymine. To determine whether Mueller-Hinton medium has sufficiently low levels of thymidine and thymine, an Enterococcus faecalis (ATCC 29212 or ATCC 33186) may be tested with sulfamethoxazole/trimethoprim disks. A zone of inhibitionmm that is essentially free of fine colonies indicates a sufficiently low level of thymidine and thymine.
INDICATIONS & USAGE
Urinary Tract Infections
Acute Otitis Media
Acute Exacerbations of Chronic Bronchitis in Adults
Shigellosis
Pneumocystis Carinii Pneumonia
Traveler's Diarrhea in Adults
SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
PRECAUTIONS
GeneralCLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
Use in the Treatment of and Prophylaxis for Pneumocystis Carinii Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS)
WARNINGS
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
Drug/Laboratory Test InteractionsCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Teratogenic EffectsPregnancy Category C
Nonteratogenic Effects
CONTRAINDICATIONS
NURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
INDICATIONSCONTRAINDICATIONSGERIATRIC USE
WARNINGSADVERSE REACTIONSDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY: Geriatric Pharmacokinetics
SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
WARNINGSPRECAUTIONS: Use in the Treatment of and Prophylaxis for Pneumocystis Carinii Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS)
WARNINGS
OVERDOSAGE
AcuteChronic
DOSAGE & ADMINISTRATION
Urinary Tract Infections and Shigellosis in Adults and Pediatric Patients, and Acute Otitis Media in Children
Adults
Children
Weight Dose-every 12 hourslbkgTablets
For Patients with Impaired Renal Function
Creatinine Clearance (mL/min)Recommended Dosage Regimen
Acute Exacerbations of Chronic Bronchitis in Adults
Pneumocystis Carinii Pneumonia
Treatment
Adults and Children
Weight Dose - every 6 hourslbkgTablets
Prophylaxis
Adults
Children
Body Surface AreaDoseevery 12 hours(m2)Tablets
Traveler's Diarrhea in Adults
HOW SUPPLIED
REFERENCES
-
?
-
? Kaplan SA, et al. Pharmacokinetic Profile of Trimethoprim-Sulfamethoxazole in Man. J Infect Dis. Nov 1973; 128 (Suppl): S547-S555.
-
? Varoquaux O, et al. Pharmacokinetics of the trimethoprim-sulfamethoxazole combination in the elderly. Br J Clin Pharmacol. 1985;20:575-581.
-
? Rudoy RC, Nelson JD, Haltalin KC. Antimicrobial Agents Chemother. May 1974;5:439-443.
-
? National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved StandardFourth Edition. NCCLS Document M7-A4, Vol.17, No. 2, NCCLS, Wayne, PA, January, 1997.
-
? National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved StandardSixth Edition. NCCLS Document M2-A6, Vol.17, No.1, NCCLS, Wayne, PA, January, 1997.
-
? Hardy DW, et al. A controlled trial of trimethoprim-sulfamethoxazole or aerosolized pentamidine for secondary prophylaxis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992; 327: 1842-1848.
-
? Marinella Mark A. 1999. Trimethoprim-induced hyperkalemia: An analysis of reported cases. Gerontol. 45:209-212.
-
? Margassery, S. and B. Bastani. 2002. Life threatening hyperkalemia and acidosis secondary to trimethoprim-sulfamethoxazole treatment. J. Nephrol. 14:410-414.
-
? Brumfitt W, Pursell R. Trimethoprim/Sulfamethoxazole in the Treatment of Bacteriuria in Women. J Infect Dis. Nov 1973; 128 (Suppl):S657-S663.
-
? Masur H. Prevention and treatment of Pneumocystis pneumonia. N Engl J Med. 1992; 327: 1853-1880.
-
? Recommendations for prophylaxis against Pneumocystis carinii pneumonia for adults and adolescents infected with human immunodeficiency virus. MMWR. 1992; 41(RR-4):1-11.
-
? CDC Guidelines for prophylaxis against Pneumocystis carinii pneumonia for children infected with human immunodeficiency virus. MMWR. 1991; 40(RR-2):1-13.
-
?
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Sulfamethoxazole and TrimethoprimSulfamethoxazle and Trimethoprim TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!