Tramadol Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- TRAMADOL HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- TRAMADOL HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- TRAMADOL HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
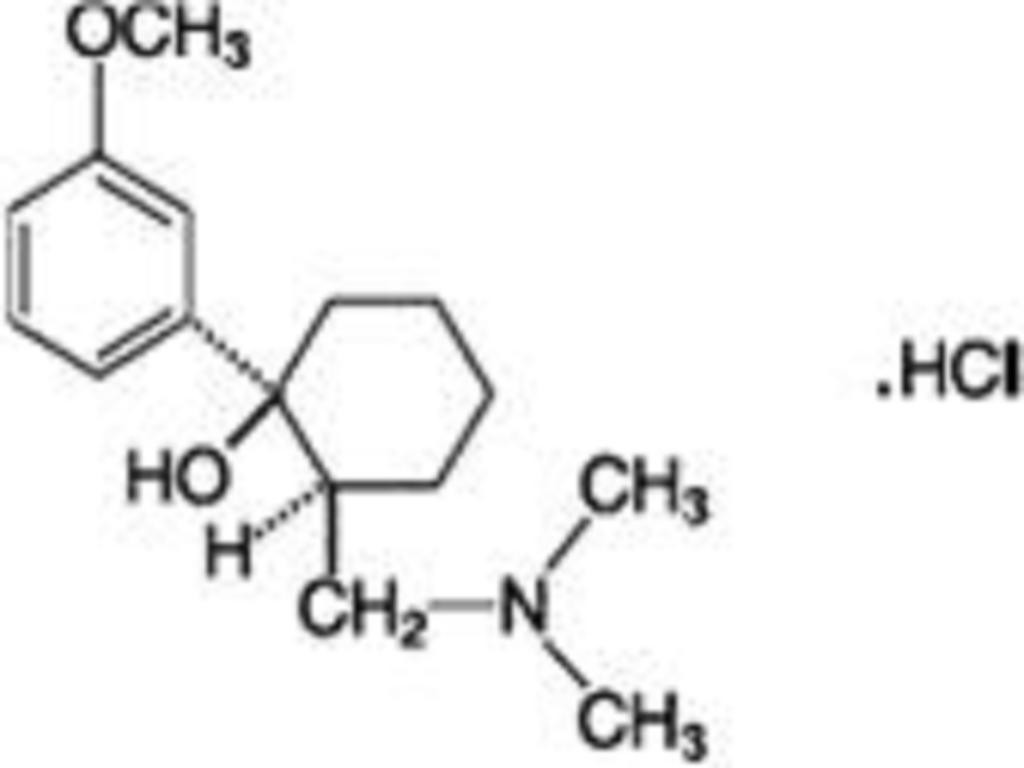
TRAMADOL HYDROCHLORIDE DESCRIPTION
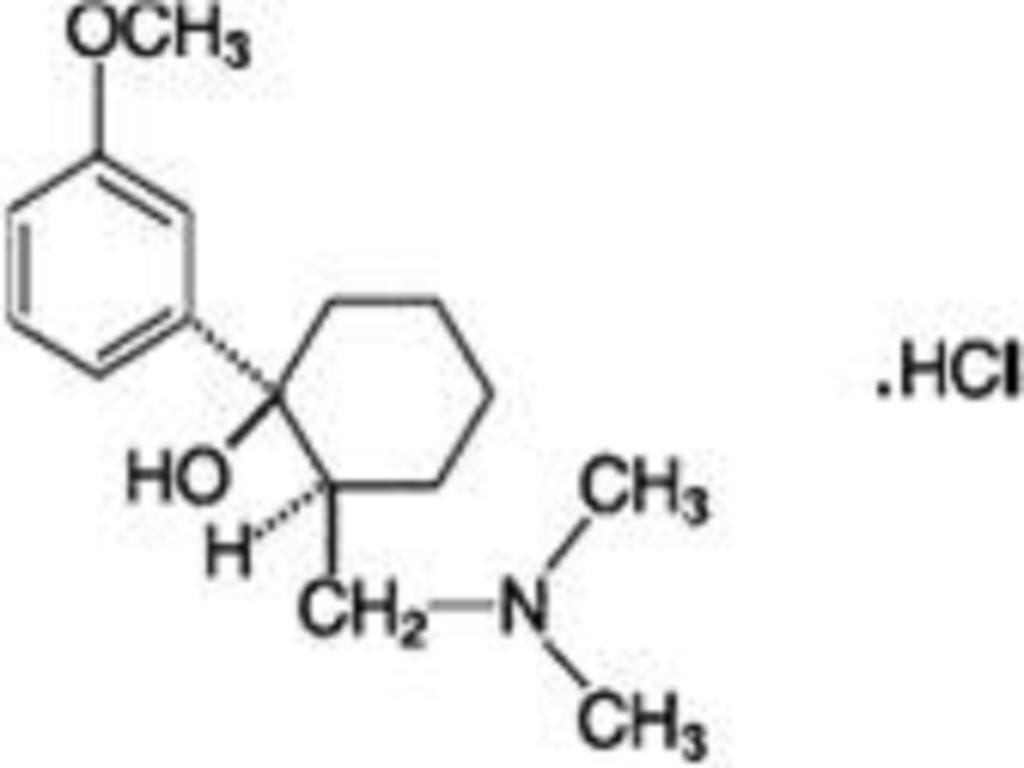
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
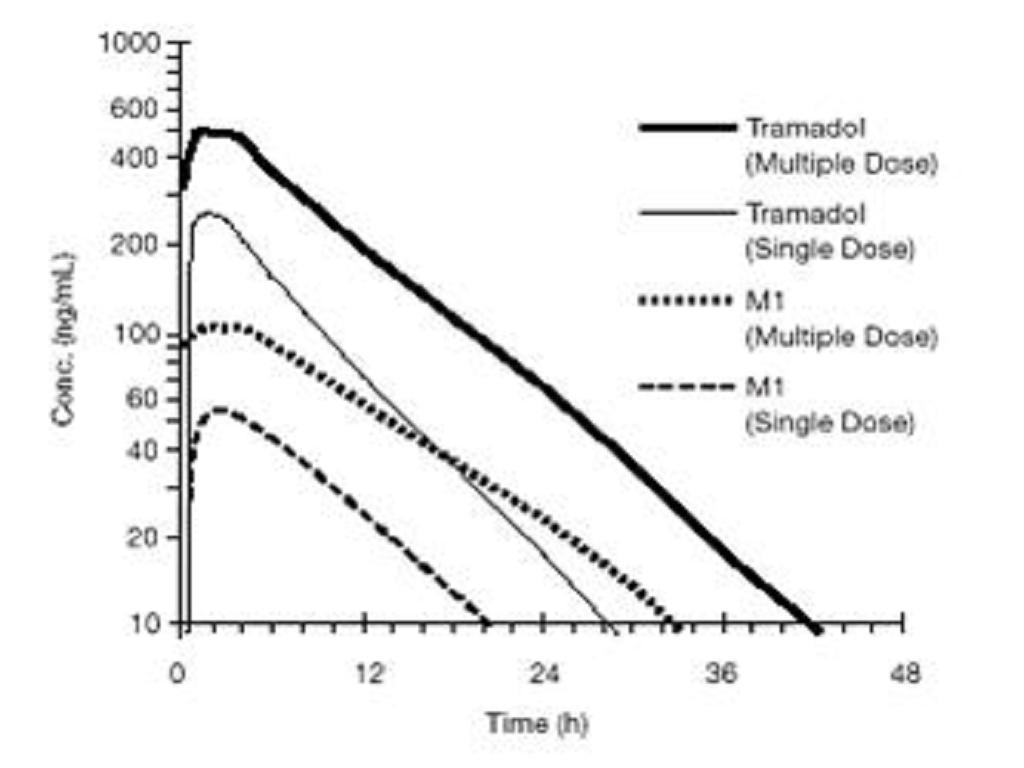
PHARMACODYNAMICSCLINICAL PHARMACOLOGY, Pharmacokinetics
PHARMACOKINETICS
CLINICAL PHARMACOLOGY, Pharmacodynamics
PRECAUTIONS, Drug Interaction
WARNINGS
Special Populations
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
CLINICAL STUDIES
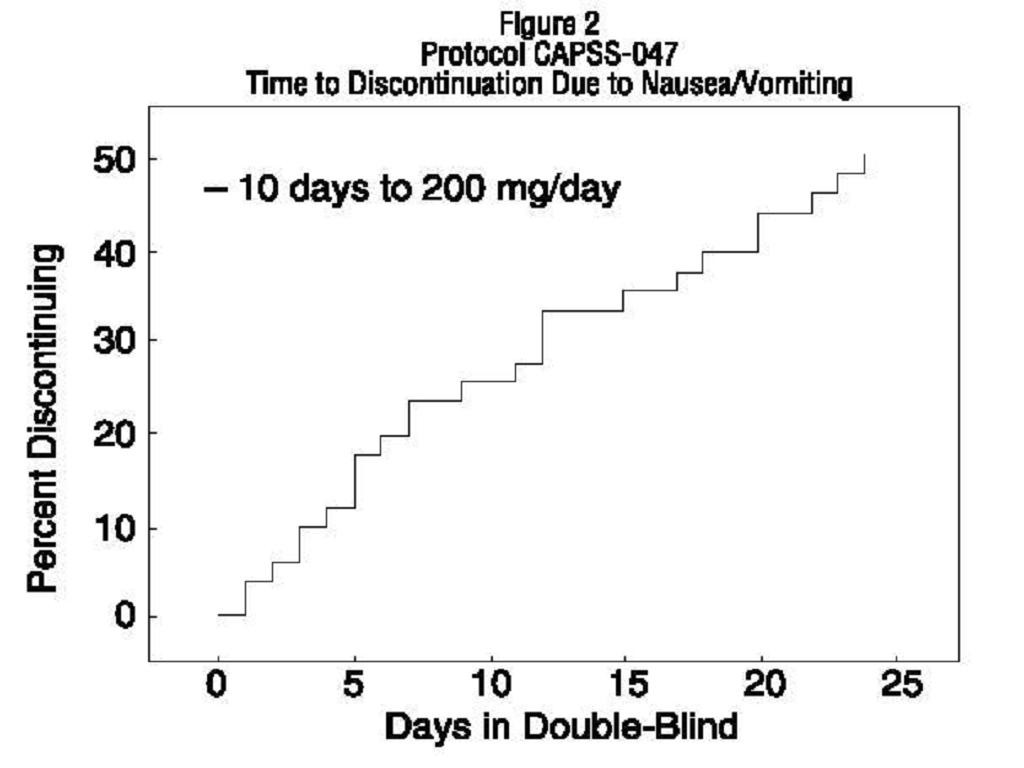
INDICATIONS & USAGE
TRAMADOL HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Seizure RiskSeizures have been reported in patients receiving Tramadol hydrochloride within the recommended dosage range. Spontaneous post-marketing reports indicate that seizure risk is increased with doses of tramadol hydrochloride above the recommended range.
Concomitant use of tramadol hydrochloride increases the seizure risk in patients taking:
-
● Selective serotonin re-uptake inhibitors (SSRI antidepressants or anorectics),
-
● Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine , promethazine, etc.), or
-
● Other opioids.
-
● MAO inhibitors (see alsoWARNINGS, Use with MAO Inhibitors and Serotonin Re-Uptake Inhibitors),
-
● Neuroleptics, or
-
● Other drugs that reduce the seizure threshold.
Suicide Risk
-
● Do not prescribe tramadol hydrochloride for patients who are suicidal or addiction-prone.
-
● Prescribe tramadol hydrochloride tablets with caution for patients who are taking tranquilizers or antidepressant drug and patients who use alcohol in excess and who suffer from emotional disturbance or depression.
Serotonin Syndrome Risk
The development of a potentially life-threatening serotonin syndrome may occur with the use of tramadol products, including tramadol hydrochloride, particularly with concomitant use of serotonergic drugs such as SSRIs, SNRIs, TCAs, MAOIs, and triptans, with drugs which impair metabolism of serotonin (including MAOIs), and with drugs which impair metabolism of tramadol (CYP2D6 and CYP3A4 inhibitors). This may occur within the recommended dose (seeCLINICAL PHARMACOLOGY, Pharmacokinetics).
Serotonin syndrome may include mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Anaphylactoid Reactions
CONTRAINDICATIONS
Respiratory Depression
WARNINGS, Seizure RiskOVERDOSAGE
Interaction With Central Nervous System (CNS) Depressants
Interactions with Alcohol and Drugs of Abuse
Increased Intracranial Pressure or Head Trauma
WARNINGS, Respiratory Depression
Use in Ambulatory Patients
Use With MAO Inhibitors and Serotonin Re-uptake Inhibitors
Misuse, Abuse and Diversion
DRUG ABUSE AND DEPENDENCEOVERDOSAGE
Risk of Overdosage
OVERDOSAGE
Withdrawal
DRUG ABUSE AND DEPENDENCE
PRECAUTIONS
Acute Abdominal ConditionsUse in Renal and Hepatic Disease
DOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATION
INFORMATION FOR PATIENTS
-
● Patients should be informed that tramadol hydrochloride may cause seizures and/or serotonin syndrome with concomitant use of serotonergic agents (including SSRIs, SNRIs, and triptans) or drugs that significantly reduce the metabolic clearance of tramadol.
-
● may impair mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
-
● Tramadol hydrochloride should not be taken with alcohol containing beverages.
-
● Tramadol hydrochloride should be used with caution when taking medications such as tranquilizers, hypnotics or other opiate containing analgesics.
-
● The patient should be instructed to inform the physician if they are pregnant, think they might become pregnant, or are trying to become pregnant (seePRECAUTIONS, Labor and Delivery).
-
● The patient should understand the single-dose and 24-hour dose limit and the time interval between doses, since exceeding these recommendations can result in respiratory depression, seizures and death.
DRUG INTERACTIONS
CLINICAL PHARMACOLOGY, Pharmacokinetics
WARNINGS, Serotonin Syndrome
WARNINGS, Serotonin Syndrome
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects: Pregnancy Category CNon-teratogenic Effects
LABOR & DELIVERY
DRUG ABUSE AND DEPENDENCENURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATIONTRAMADOL HYDROCHLORIDE ADVERSE REACTIONS
Body as a Whole:
Cardiovascular:
Central Nervous System:
Gastrointestinal:
Musculoskeletal:
Skin:
Special Senses:
Urogenital:
Body as a Whole:
Cardiovascular:
Central Nervous System:WARNINGS
Respiratory:
Skin:
Special Senses:
Urogenital:
Cardiovascular:
Central Nervous System:
Gastrointestinal:
Laboratory Abnormalities:
Sensory:
DRUG ABUSE AND DEPENDENCE
AbuseTramadol has mu-opioid agonist activity. Tramadol hydrochloride tablets can be abused and may be subject to criminal diversion.
Dependence
WARNINGS, Withdrawal
OVERDOSAGE
WARNINGS, Misuse, Abuse, and DiversionWhile naloxone will reverse some, but not all, symptoms caused by overdosage with tramadol, the risk of seizures is also increased with naloxone administration. In animals convulsions following the administration of toxic doses of tramadol hydrochloride tablets could be suppressed with barbiturates or benzodiazepines but were increased with naloxone. Naloxone administration did not change the lethality of an overdose in mice. Hemodialysis is not expected to be helpful in an overdose because it removes less than 7% of the administered dose in a 4-hour dialysis period.
DOSAGE & ADMINISTRATION
Adults (17 years of age and over)Individualization of Dose
-
● In all patients with creatinine clearance less than 30 mL/min, it is recommended that the dosing interval of tramadol hydrochloride tablets be increased to 12 hours, with a maximum daily dose of 200 mg. Since only 7% of an administered dose is removed by hemodialysis, dialysis patients can receive their regular dose on the day of dialysis.
-
● The recommended dose for adult patients with cirrhosis is 50 mg every 12 hours.
-
● In general, dose selection for an elderly patient over 65 years old should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy. For elderly patients over 75 years old, total dose should not exceed 300 mg/day.
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Tramadol HydrochlorideTramadol Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!