SHISEIDO CO., LTD.
Shiseido Advanced Hydro-Liquid Compact Foundation
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
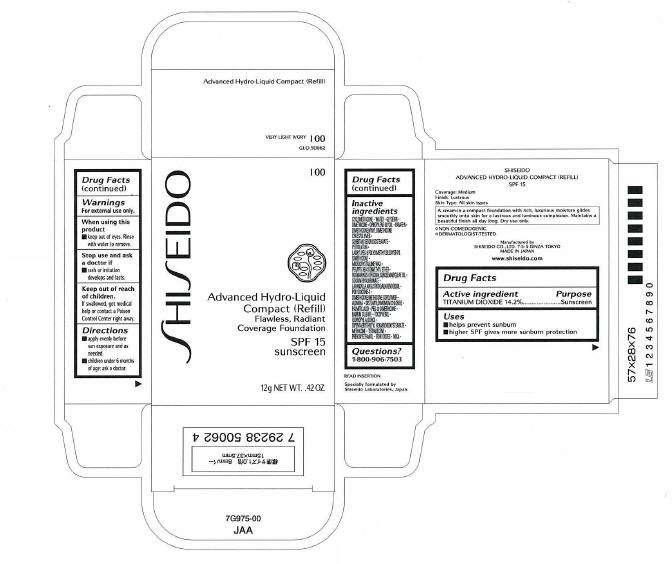
Drug Facts
Active ingredient
TITANIUM DIOAXIDE 14.2%
Purpose
Sunscreen
ADVANCED HYDRO-LIQUID COMPACT (REFILL) Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
For external use only.
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply evenly before sun exposure and as needed
- children under 6 months of age: ask a doctor
Inactive ingredients
CYCLOMETHICONE, WATER, GLYCERIN, DIMETHICONE, DIPROPYLENE GLYCOL, PARAFFIN, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, SORBITAN SESQUIISOSTEARATE, PETROLATUM, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, MICROCRYSTALLINE WAX, PEG/PPG-36/41 DIMETHYL ETHER, ROSMARINUS OFFICINALIS (ROSEMARY) LEAF OIL, SODIUM HYALURONATE, LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL, POLYSILICONE-2, DIMETHICONE/METHICONE COPOLYMER, ALUMINA, DISTEARYLDIMONIUM CHLORIDE, PALMITIC ACID, PEG-10 DIMETHICONE, BARIUM SULFATE, TOCOPHEROL, ISOPROPYL ALCOHOL, DIPENTAERYTHRITYL HEXAHYDROXYSTEARATE, METHICONE, TETRADECENE, PHENOXYETHANOL, IRON OXIDES, MICA,
Questions ?
1-800-906-7503
PRINCIPAL DISPLAY PANEL - 12g Carton
SHISEIDO
I00
Advanced Hydro-Liquid
Compact (Refill)
Flawless, Radiant
Coverage Foundation
SPF 15
sunscreen
12g NET WT. .42 OZ.
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-308 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-308-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-309 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-309-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-310 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-310-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-311 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-311-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-312 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-312-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-313 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-313-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-314 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-314-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-315 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-315-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-316 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-316-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-317 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-317-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
ADVANCED HYDRO-LIQUID COMPACT (REFILL)
Titanium dioxide POWDER
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52685-318 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
1.7 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
12 in 1 TRAY |
|
|
|
2 |
NDC:52685-318-30 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2009-09-01 |
|
|
