Allopurinol
FULL PRESCRIBING INFORMATION: CONTENTS*
- ALLOPURINOL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ALLOPURINOL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ALLOPURINOL ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ALLOPURINOL DESCRIPTION
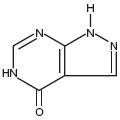
CLINICAL PHARMACOLOGY
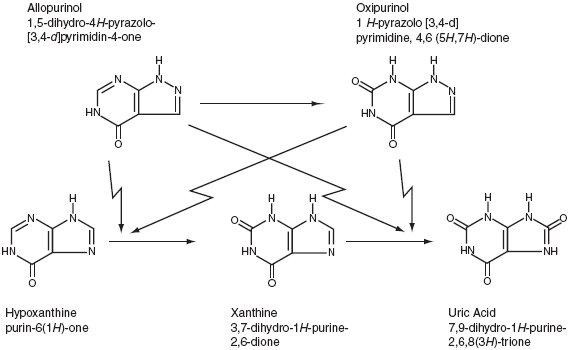
INDICATIONS AND USAGE).
INDICATIONS & USAGE
CLINICAL PHARMACOLOGY,CONTRAINDICATIONS,WARNINGS, andPRECAUTIONS).
ALLOPURINOL CONTRAINDICATIONS
WARNINGS
CLINICAL PHARMACOLOGY).
PRECAUTIONS
GeneralInformation for Patients
Laboratory Tests
WARNINGS).
Drug Interactions
CLINICAL PHARMACOLOGY).
Drug/Laboratory Test Interactions
Pregnancy
Teratogenic Effects:
Nursing Mothers
Pediatric Use
INDICATIONS AND USAGE, andDOSAGE AND ADMINISTRATION).
ALLOPURINOL ADVERSE REACTIONS
PRECAUTIONSandDOSAGE AND ADMINISTRATION).WARNINGS). Some patients with the most severe reaction also had fever, chills, arthralgias, cholestatic jaundice, eosinophilia and mild leukocytosis or leukopenia. Among 55 patients with gout treated with allopurinol for 3 to 34 months (average greater than 1 year) and followed prospectively, Rundles observed that 3% of patients developed a type of drug reaction which was predominantly a pruritic maculopapular skin eruption, sometimes scaly or exfoliative. However, with current usage, skin reactions have been observed less frequently than 1%. The explanation for this decrease is not obvious. The incidence of skin rash may be increased in the presence of renal insufficiency. The frequency of skin rash among patients receiving ampicillin or amoxicillin concurrently with allopurinol has been reported to be increased (seePRECAUTIONS).
Most Common Reactions* Probably Causally Related
ADVERSE REACTIONSintroduction,INDICATIONS AND USAGE,PRECAUTIONS, andDOSAGE AND ADMINISTRATION).
Incidence Less Than 1% Probably Causally Related
PRECAUTIONS)
Incidence Less Than 1% Causal Relationship Unknown
OVERDOSAGE
DOSAGE & ADMINISTRATION
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

AllopurinolALLOPURINOL TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!