Amlodipine Besylate
FULL PRESCRIBING INFORMATION: CONTENTS*
- AMLODIPINE BESYLATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS AND METABOLISM
- PHARMACODYNAMICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- AMLODIPINE BESYLATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- AMLODIPINE BESYLATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
AMLODIPINE BESYLATE DESCRIPTION
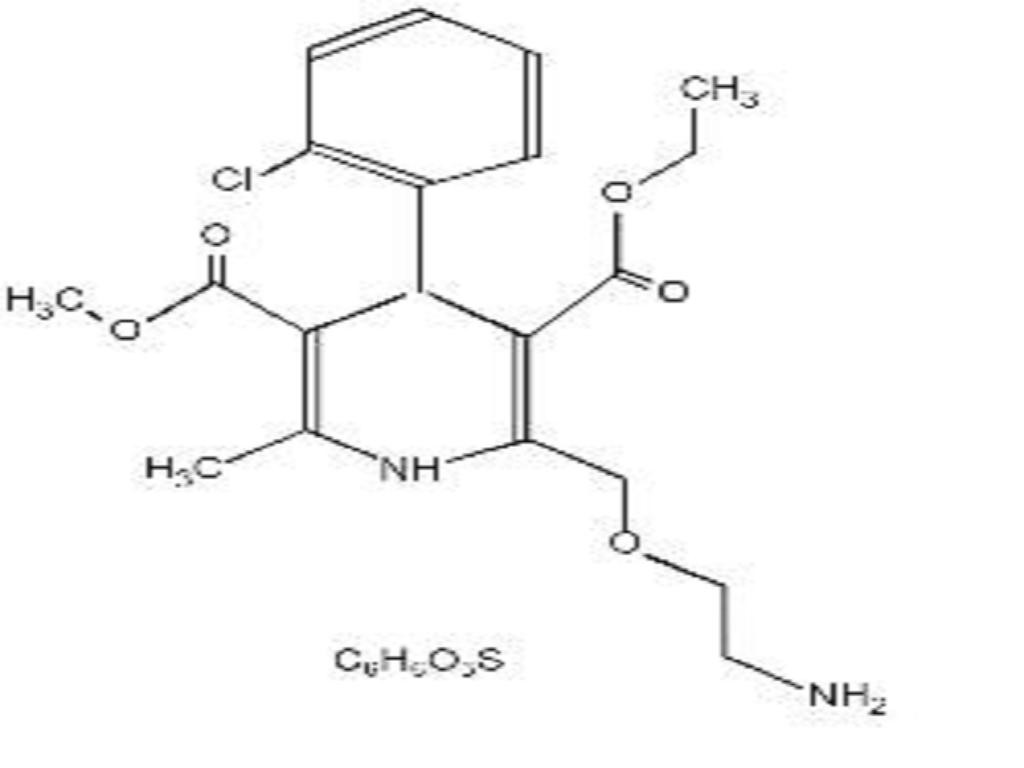
CLINICAL PHARMACOLOGY
Mechanism of ActionPHARMACOKINETICS AND METABOLISM
Pediatric Patients
PHARMACODYNAMICS
HemodynamicsElectrophysiologic Effects:
CLINICAL STUDIES
Effects in HypertensionAdult Patients:
Pediatric Patients:
Effects in Chronic Stable Angina:
Effects in Vasospastic Angina:
Studies in Patients with Congestive Heart Failure:
INDICATIONS & USAGE
HypertensionChronic Stable Angina
Vasospastic Angina (Prinzmetal's or Variant Angina)
AMLODIPINE BESYLATE CONTRAINDICATIONS
WARNINGS
Increased Angina and/or Myocardial Infarction:PRECAUTIONS
General:Use in Patients with Congestive Heart Failure:
Beta-Blocker Withdrawal:
Patients with Hepatic Failure:
DRUG INTERACTIONS
Effect of other agents on amlodipine.
Effect of amlodipine on other agents.
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Category C:NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
AMLODIPINE BESYLATE ADVERSE REACTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adults:Children:
Coadministration with Other Antihypertensive and/or Antianginal Drugs:
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Amlodipine BesylateAmlodipine Besylate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!