Amlodipine
Macleods Pharmaceuticals Limited
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use amlodipine besylate tablets USP safely and effectively. See full prescribing information for amlodipine besylate tablets USP. Amlodipine Besylate Tablets USP for oral administration Initial U.S. Approval: 1987INDICATIONS AND USAGE1.11.2DOSAGE AND ADMINISTRATION2.12.12.2Important Limitation:DOSAGE FORMS AND STRENGTHS3CONTRAINDICATIONS4WARNINGS AND PRECAUTIONS Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. However, acute hypotension is unlikely.(5.1) Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine besylate, particularly in patients with severe obstructive coronary artery disease. (5.2) Titrate slowly in patients with severe hepatic impairment. (5.4) Side Effects6 To report SUSPECTED ADVERSE REACTIONS, contact Macleods Pharma USA, Inc. at 1-888-943-3210 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch . DRUG INTERACTIONS7.7USE IN SPECIFIC POPULATIONS8.18.38.4
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 INDICATIONS & USAGE
- 2 DOSAGE & ADMINISTRATION
- 3 DOSAGE FORMS & STRENGTHS
- 4 AMLODIPINE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 AMLODIPINE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 AMLODIPINE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS & USAGE
1.1 Hypertension
1.2 Coronary Artery Disease (CAD)
Chronic Stable Angina
Amlodipine besylate tablet USP is indicated for the symptomatic treatment of chronic stable angina. Amlodipine besylate may be used alone or in combination with other antianginal agents.
Vasospastic Angina (Prinzmetal's or Variant Angina)
Angiographically Documented CAD
2 DOSAGE & ADMINISTRATION
2.1 Adults
AnginaAdverse Reactions (6)
Coronary artery disease:Clinical Studies (14.4)
2.2 Children
[see Clinical Pharmacology (12.4), Clinical Studies (14.1)].
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
5.2 Increased Angina or Myocardial Infarction
5.3 Patients with Hepatic Failure
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
|
Amlodipine |
Placebo |
|||
|
2.5mg N=275 |
5 mg N=296 |
10 mg N=268 |
N=520 |
|
|
Edema |
1.8 |
3.0 |
10.8 |
0.6 |
|
Dizziness |
1.1 |
3.4 |
3.4 |
1.5 |
|
Flushing |
0.7 |
1.4 |
2.6 |
0.0 |
|
Palpitation |
0.7 |
1.4 |
4.5 |
0.6 |
|
Amlodipine besylate (%) |
Placebo (%) |
|
|
(N=1730) |
(N=1250) |
|
|
Fatigue |
4.5 |
2.8 |
|
Nausea |
2.9 |
1.9 |
|
Abdominal Pain |
1.6 |
0.3 |
|
Somnolence |
1.4 |
0.6 |
|
Amlodipine besylate |
Placebo |
|||
|
Male=% (N=1218) |
Female=% (N=512) |
Male=% (N=914) |
Female=% (N=336) |
|
| Edema |
5.6 |
14.6 |
1.4 |
5.1 |
| Flushing |
1.5 |
4.5 |
0.3 |
0.9 |
| Palpitations |
1.4 |
3.3 |
0.9 |
0.9 |
| Somnolence |
1.3 |
1.6 |
0.8 |
0.3 |
Cardiovascular:
Central and Peripheral Nervous System:
Gastrointestinal:
General:1
Musculoskeletal System:1
Psychiatric:1
Respiratory System:1
Skin and Appendages:11
Special Senses:
System:
Autonomic Nervous System:
Metabolic and Nutritional:
Hemopoietic:
1
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 In Vitro Data
7.2 Cimetidine
7.3 Grapefruit Juice
7.4 Magnesium and Aluminum Hydroxide Antacid
7.5 Sildenafil
7.6 Atorvastatin
7.7 Simvastatin
7.8 Digoxin
7.9 Ethanol (Alcohol)
7.10 Warfarin
7.11 CYP3A4 Inhibitors
7.12 CYP3A4 Inducers
7.13 Cyclosporine
7.14 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
222
2
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
Dosage and Administration (2.1)
10 OVERDOSAGE
2
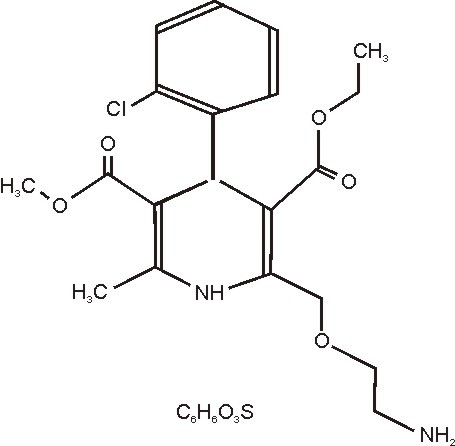
11 DESCRIPTION
202525663
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics and Metabolism
12.4 Pediatric Patients
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
2323
32
3
14 CLINICAL STUDIES
14.1 Effects in Hypertension
Adult Patients
Pediatric Patients
14.2 Effects in Chronic Stable Angina
14.3 Effects in Vasospastic Angina
14.4 Effects in Documented Coronary Artery Disease
Figure 1 - Kaplan-Meier Analysis of Composite Clinical Outcomes for amlodipine versus Placebo
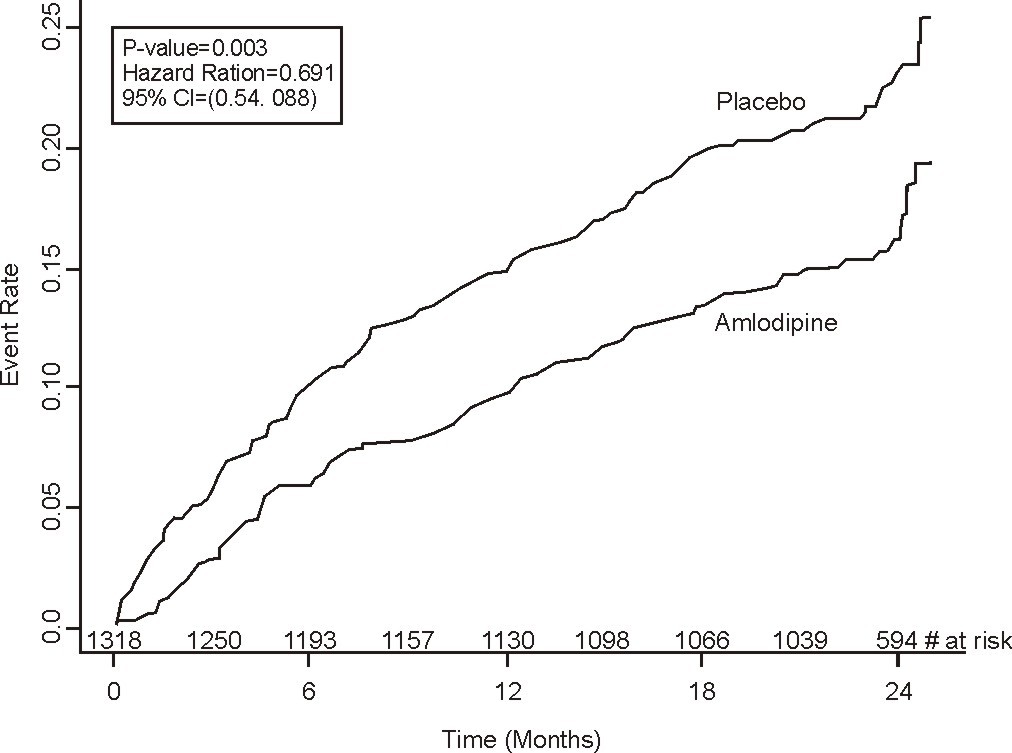
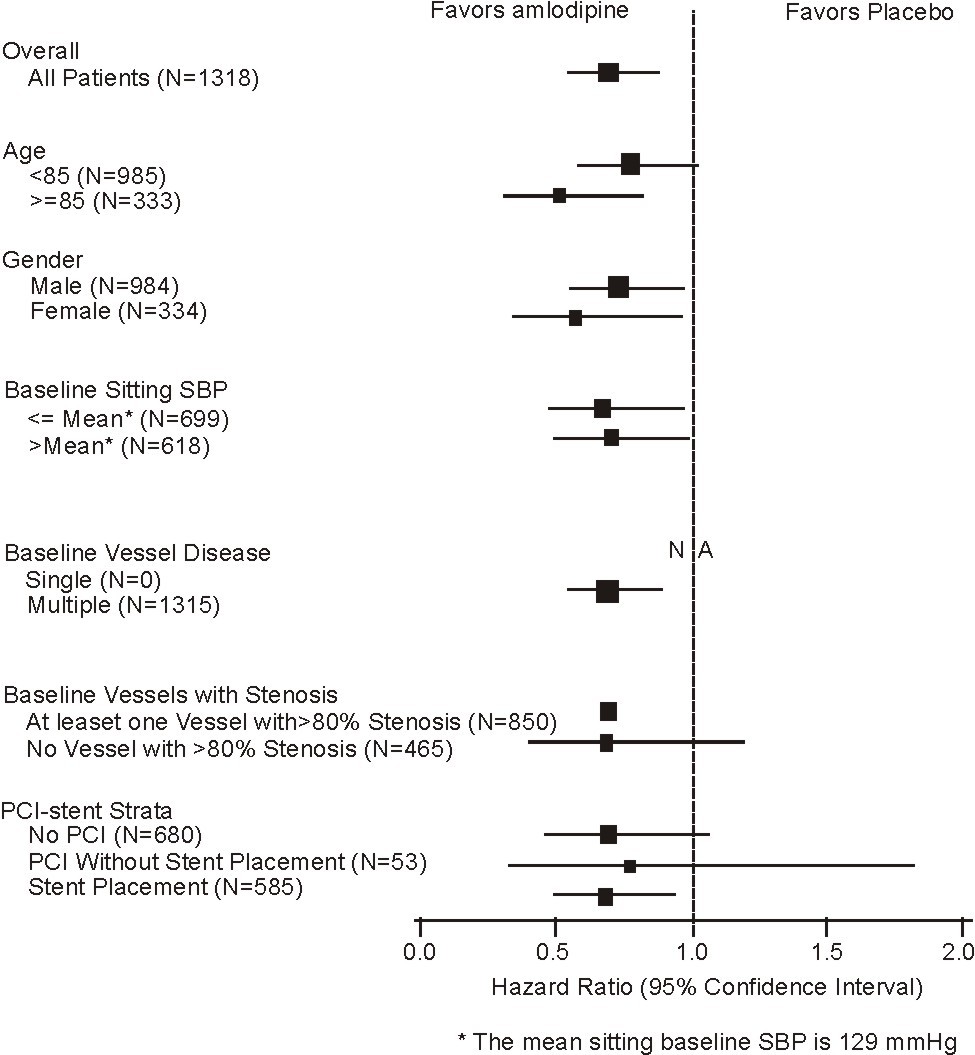
Figure 2 – Effects on Primary Endpoint of amlodipine versus Placebo across Sub-Groups
|
Clinical Outcomes N (%) |
A
mlodipine besylate
(N=663) |
Placebo (N=655) |
Risk Reduction (p-value) |
|
Composite CV Endpoint
|
110 (16.6) |
151 (23.1) |
31% (0.003) |
Hospitalization for Angina |
51 (7.7) |
84 (12.8) |
42% (0.002) |
Coronary Revascularization |
78 (11.8) |
103 (15.7) |
27% (0.033) |
14.5 Studies in Patients with Heart Failure
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 5 mg Tablets
16.2 10 mg Tablets
16.3 Storage
17 PATIENT COUNSELING INFORMATION
Amlodipine besylate tablets USPamlodipine besylate tabletsamlodipine besylate tabletsamlodipine besylate tablet
What is amlodipine besylate?
Amlodipine besylate USP
High Blood Pressure (hypertension)
Amlodipine besylate
Angina
Amlodipine besylate
Who should not use amlodipine besylate tablets?
amlodipine besylate tabletsamlodipine besylate tablets
What should I tell my doctor before taking amlodipine besylate tablets ?
amlodipine besylate tablet
amlodipine besylate tablets
amlodipine besylate tablets
amlodipine besylate tablet
amlodipine besylate tablet
amlodipine besylate tablet
amlodipine besylate tablet
amlodipine besylate tablet
amlodipine besylate tablet
amlodipine besylate tablet
What should I avoid while taking amlodipine besylate tablets?
Do notamlodipine besylate tablet
Do not
What are the possible side effects of amlodipine besylate tablet?
Amlodipine besylate tablets
amlodipine besylate tablet
amlodipine besylate tablet
How do I store amlodipine besylate tablets?
amlodipine besylate tabletsamlodipine besylate tabletsamlodipine besylate tablets
General advice about amlodipine besylate tablets
amlodipine besylate tabletsamlodipine besylate tablets
amlodipine besylate tabletsMacleods Pharma USA, Inc. at 1-888-943-3210.
Manufactured for:
Manufacturedby:
Revised : February 2013
PACKAGE LABEL PRINCIPAL DISPLAY PANEL


AmlodipineAmlodipine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AmlodipineAmlodipine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!