ANASTROZOLE
Sun Pharmaceutical Industries Limited
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use anastrozole tablets safely and effectively. See full prescribing information for Anastrozole Tablets. Anastrozole tablet for oral useInitial U.S. Approval: 1995 INDICATIONS AND USAGE Adjuvant treatment of postmenopausal women with hormone receptor-positive early breast cancer (1.1) First-line treatment of postmenopausal women with hormone receptor-positive or hormone receptor unknown locally advanced or metastatic breast cancer (1.2) Treatment of advanced breast cancer in postmenopausal women with disease progression following tamoxifen therapy. Patients with ER-negative disease and patients who did not respond to previous tamoxifen therapy rarely responded to anastrozole (1.3) CONTRAINDICATIONS Women of premenopausal endocrine status, including pregnant women (4.1, 8.1) Patients with demonstrated hypersensitivity to anastrozole tablets or any excipient (4.2) WARNINGS AND PRECAUTIONS In women with preexisting ischemic heart disease, an increased incidence of ischemic cardiovascular events occurred with anastrozole use compared to tamoxifen use. Consider risks and benefits. (5.1, 6.1) Decreases in bone mineral density may occur. Consider bone mineral density monitoring. (5.2, 6.1) Increases in total cholesterol may occur. Consider cholesterol monitoring. (5.3, 6.1) Side EffectsTo report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchDRUG INTERACTIONS Tamoxifen: Do not use in combination with anastrozole. No additional benefit seen over tamoxifen monotherapy (7.1, 14.1). Estrogen-containing products: Combination use may diminish activity of anastrozole (7.2). USE IN SPECIFIC POPULATIONS Pediatric patients: Efficacy has not been demonstrated for pubertal boys of adolescent age with gynecomastia or girls with McCune-Albright Syndrome and progressive precocious puberty. (8.4)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ANASTROZOLE INDICATIONS AND USAGE
- 2 ANASTROZOLE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ANASTROZOLE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ANASTROZOLE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 ANASTROZOLE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- FDA-Approved Patient Labeling
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 1MG
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Adjuvant Treatment
1.2 First-Line Treatment
1.3 Second-Line Treatment
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The dose of anastrozole tablet is one 1 mg tablet taken once a day. For patients with advanced breast cancer, anastrozole tablets should be continued until tumor progression. Anastrozole tablets can be taken with or without food.
For adjuvant treatment of early breast cancer in postmenopausal women, the optimal duration of therapy is unknown. In the ATAC trial, anastrozole tablet was administered for five years. [see Clinical Studies (14.1)]
No dosage adjustment is necessary for patients with renal impairment or for elderly patients. [see Use in Specific Populations (8.6)]
2.2 Patients with Hepatic Impairment
[see Use in Specific Populations (8.7)]
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Pregnancy and Premenopausal Women
Anastrozole tablets [see Use in Specific Populations (8.1)]
4.2 Hypersensitivity
[see Adverse Reactions (6.2)]
5 WARNINGS AND PRECAUTIONS
5.1 Ischemic Cardiovascular Events
In women[see Adverse Reactions (6.1)]
5.2 Bone Effects
Results from the ATAC trial bone substudy at 12 and 24 months demonstrated that patients receiving anastrozole tablets had a mean decrease in both lumbar spine and total hip bone mineral density (BMD) compared to baseline. Patients receiving tamoxifen had a mean increase in both lumbar spine and total hip BMD compared to baseline [see Adverse Reactions (6.1)].
5.3 Cholesterol
[see Adverse Reactions (6.1)]
6 ADVERSE REACTIONS
[see Adverse Reactions (6.2)]
6.1 Clinical Trials Experience
Adjuvant Therapy
[see Clinical Studies (14.1)]
Body system and adverse reactions by COSTART  |
Anastrozole 1 mg (N  |
Tamoxifen 20 mg (N  |
|---|---|---|
|
Body as a whole
|
||
| Asthenia |
575 (19) |
544 (18) |
| Pain |
533 (17) |
485 (16) |
| Back pain |
321 (10) |
309 (10) |
| Headache |
314 (10) |
249 (8) |
| Abdominal pain |
271 (9) |
276 (9) |
| Infection |
285 (9) |
276 (9) |
| Accidental injury |
311 (10) |
303 (10) |
| Flu syndrome |
175 (6) |
195 (6) |
| Chest pain |
200 (7) |
150 (5) |
| Neoplasm |
162 (5) |
144 (5) |
| Cyst |
138 (5) |
162 (5) |
|
Cardiovascular
|
||
| Vasodilatation |
1104 (36) |
1264 (41) |
| Hypertension |
402 (13) |
349 (11) |
|
Digestive
|
||
| Nausea |
343 (11) |
335 (11) |
| Constipation |
249 (8) |
252 (8) |
| Diarrhea |
265 (9) |
216 (7) |
| Dyspepsia |
206 (7) |
169 (6) |
| Gastrointestinal disorder |
210 (7) |
158 (5) |
|
Hemic and lymphatic
|
||
| Lymphedema |
304 (10) |
341 (11) |
| Anemia |
113 (4) |
159 (5) |
|
Metabolic and nutritional
|
||
| Peripheral edema |
311 (10) |
343 (11) |
| Weight gain |
285 (9) |
274 (9) |
| Hypercholesterolemia |
278 (9) |
108 (3.5) |
|
Musculoskeletal
|
||
| Arthritis |
512 (17) |
445 (14) |
| Arthralgia |
467 (15) |
344 (11) |
| Osteoporosis |
325 (11) |
226 (7) |
| Fracture |
315 (10) |
209 (7) |
| Bone pain |
201 (7) |
185 (6) |
| Arthrosis |
207 (7) |
156 (5) |
| Joint Disorder |
184 (6) |
160 (5) |
| Myalgia |
179 (6) |
160 (5) |
|
Nervous system
|
||
| Depression |
413 (13) |
382 (12) |
| Insomnia |
309 (10) |
281 (9) |
| Dizziness |
236 (8) |
234 (8) |
| Anxiety |
195 (6) |
180 (6) |
| Paresthesia |
215 (7) |
145 (5) |
|
Respiratory
|
||
| Pharyngitis |
443 (14) |
422 (14) |
| Cough increased |
261 (8) |
287 (9) |
| Dyspnea |
234 (8) |
237 (8) |
| Sinusitis |
184 (6) |
159 (5) |
| Bronchitis |
167 (5) |
153 (5) |
|
Skin and appendages
|
||
| Rash |
333 (11) |
387 (13) |
| Sweating |
145 (5) |
177 (6) |
|
Special Senses
|
||
| Cataract Specified |
182 (6) |
213 (7) |
|
Urogenital
|
||
| Leukorrhea |
86 (3) |
286 (9) |
| Urinary tract infection |
244 (8) |
313 (10) |
| Breast pain |
251 (8) |
169 (6) |
| Breast Neoplasm |
164 (5) |
139 (5) |
| Vulvovaginitis |
194 (6) |
150 (5) |
Vaginal Hemorrhage |
122 (4) |
180 (6) |
| Vaginitis |
125 (4) |
158 (5) |
| Anastrozole N=3092 (%) |
Tamoxifen N=3094 (%) |
Odds-ratio | 95% CI | |
|---|---|---|---|---|
| Hot Flashes |
1104 (36) |
1264 (41) |
0.8 |
0.73 to 0.89 |
Musculoskeletal Events |
1100 (36) |
911 (29) |
1.32 |
1.19 to 1.47 |
| Fatigue/Asthenia |
575 (19) |
544 (18) |
1.07 |
0.94 to 1.22 |
| Mood Disturbances |
597 (19) |
554 (18) |
1.1 |
0.97 to 1.25 |
| Nausea and Vomiting |
393 (13) |
384 (12) |
1.03 |
0.88 to 1.19 |
| All Fractures |
315 (10) |
209 (7) |
1.57 |
1.3 to 1.88 |
| Fractures of Spine, Hip, or Wrist |
133 (4) |
91 (3) |
1.48 |
1.13 to 1.95 |
| Wrist/Colles’ fractures |
67 (2) |
50 (2) |
||
| Spine fractures |
43 (1) |
22 (1) |
||
| Hip fractures |
28 (1) |
26 (1) |
||
| Cataracts |
182 (6) |
213 (7) |
0.85 |
0.69 to 1.04 |
| Vaginal Bleeding |
167 (5) |
317 (10) |
0.5 |
0.41 to 0.61 |
| Ischemic Cardiovascular Disease |
127 (4) |
104 (3) |
1.23 |
0.95 to 1.6 |
| Vaginal Discharge |
109 (4) |
408 (13) |
0.24 |
0.19 to 0.3 |
| Venous Thromboembolic events |
87 (3) |
140 (5) |
0.61 |
0.47 to 0.8 |
| Deep Venous Thromboembolic Events |
48 (2) |
74 (2) |
0.64 |
0.45 to 0.93 |
| Ischemic Cerebrovascular Event |
62 (2) |
88 (3) |
0.7 |
0.5 to 0.97 |
Endometrial Cancer |
4 (0.2) |
13 (0.6) |
0.31 |
0.1 to 0.94 |
Bone Mineral Density Findings
Cholesterol
Other Adverse Reactions
10-year median follow-up Safety Results from the ATAC Trial
- Results are consistent with the previous analyses.
- Serious adverse reactions were similar between anastrozole (50%) and tamoxifen (51%).
- Cardiovascular events were consistent with the known safety profiles of anastrozole and tamoxifen.
- The cumulative incidences of all first fractures (both serious and non-serious, occurring either during or after treatment) was higher in the anastrozole group (15%) compared to the tamoxifen group (11%). This increased first fracture rate during treatment did not continue in the post-treatment follow-up period.
- The cumulative incidence of new primary cancers was similar in the anastrozole group (13.7%) compared to the tamoxifen group (13.9%). Consistent with the previous analyses, endometrial cancer was higher in the tamoxifen group (0.8%) compared to the anastrozole group (0.2%).
- The overall number of deaths (during or off-trial treatment) was similar between the treatment groups. There were more deaths related to breast cancer in the tamoxifen than in the anastrozole treatment group.
First-Line Therapy
| Number (%) of subjects | ||
|---|---|---|
Body system Adverse Reaction |
Anastrozole (N=506) |
Tamoxifen (N=511) |
|
Whole body
|
||
| Asthenia |
83 (16) |
81 (16) |
| Pain |
70 (14) |
73 (14) |
| Back pain |
60 (12) |
68 (13) |
| Headache |
47 (9) |
40 (8) |
| Abdominal pain |
40 (8) |
38 (7) |
| Chest pain |
37 (7) |
37 (7) |
| Flu syndrome |
35 (7) |
30 (6) |
| Pelvic pain |
23 (5) |
30 (6) |
|
Cardiovascular
|
||
| Vasodilation |
128 (25) |
106 (21) |
| Hypertension |
25 (5) |
36 (7) |
|
Digestive
|
||
| Nausea |
94 (19) |
106 (21) |
| Constipation |
47 (9) |
66 (13) |
| Diarrhea |
40 (8) |
33 (6) |
| Vomiting |
38 (8) |
36 (7) |
| Anorexia |
26 (5) |
46 (9) |
|
Metabolic and Nutritional
|
||
| Peripheral edema |
51 (10) |
41 (8) |
|
Musculoskeletal
|
||
| Bone pain |
54 (11) |
52 (10) |
|
Nervous
|
||
| Dizziness |
30 (6) |
22 (4) |
| Insomnia |
30 (6) |
38 (7) |
| Depression |
23 (5) |
32 (6) |
| Hypertonia |
16 (3) |
26 (5) |
|
Respiratory
|
||
| Cough increased |
55 (11) |
52 (10) |
| Dyspnea |
51 (10) |
47 (9) |
| Pharyngitis |
49 (10) |
68 (13) |
|
Skin and appendages
|
||
| Rash |
38 (8) |
34 (8) |
|
Urogenital
|
||
| Leukorrhea |
9 (2) |
31 (6) |
| Number (n) and Percentage of Patients | ||
|---|---|---|
Adverse Reaction |
Anastrozole 1 mg (N = 506) n (%) |
Tamoxifen Citrate 20 mg (N = 511) n (%) |
| Depression |
23 (5) |
32 (6) |
| Tumor Flare |
15 (3) |
18 (4) |
Thromboembolic Disease |
18 (4) |
33 (6) |
Venous |
5 |
15 |
Coronary and Cerebral |
13 |
19 |
| Gastrointestinal Disturbance |
170 (34) |
196 (38) |
| Hot Flushes |
134 (26) |
118 (23) |
| Vaginal Dryness |
9 (2) |
3 (1) |
| Lethargy |
6 (1) |
15 (3) |
| Vaginal Bleeding |
5 (1) |
11 (2) |
| Weight Gain |
11 (2) |
8 (2) |
Adverse Reaction |
Anastrozole 1 mg (N=262) |
Anastrozole 10 mg (N=246) |
Megestrol Acetate 160 mg (N=253) |
|||
|---|---|---|---|---|---|---|
| |
n
|
%
|
n
|
%
|
n
|
%
|
| Asthenia |
42 |
(16) |
33 |
(13) |
47 |
(19) |
| Nausea |
41 |
(16) |
48 |
(20) |
28 |
(11) |
| Headache |
34 |
(13) |
44 |
(18) |
24 |
(9) |
| Hot Flashes |
32 |
(12) |
29 |
(11) |
21 |
(8) |
| Pain |
28 |
(11) |
38 |
(15) |
29 |
(11) |
| Back Pain |
28 |
(11) |
26 |
(11) |
19 |
(8) |
| Dyspnea |
24 |
(9) |
27 |
(11) |
53 |
(21) |
| Vomiting |
24 |
(9) |
26 |
(11) |
16 |
(6) |
| Cough Increased |
22 |
(8) |
18 |
(7) |
19 |
(8) |
| Diarrhea |
22 |
(8) |
18 |
(7) |
7 |
(3) |
| Constipation |
18 |
(7) |
18 |
(7) |
21 |
(8) |
| Abdominal Pain |
18 |
(7) |
14 |
(6) |
18 |
(7) |
| Anorexia |
18 |
(7) |
19 |
(8) |
11 |
(4) |
| Bone Pain |
17 |
(6) |
26 |
(12) |
19 |
(8) |
| Pharyngitis |
16 |
(6) |
23 |
(9) |
15 |
(6) |
| Dizziness |
16 |
(6) |
12 |
(5) |
15 |
(6) |
| Rash |
15 |
(6) |
15 |
(6) |
19 |
(8) |
| Dry Mouth |
15 |
(6) |
11 |
(4) |
13 |
(5) |
| Peripheral Edema |
14 |
(5) |
21 |
(9) |
28 |
(11) |
| Pelvic Pain |
14 |
(5) |
17 |
(7) |
13 |
(5) |
| Depression |
14 |
(5) |
6 |
(2) |
5 |
(2) |
| Chest Pain |
13 |
(5) |
18 |
(7) |
13 |
(5) |
| Paresthesia |
12 |
(5) |
15 |
(6) |
9 |
(4) |
| Vaginal Hemorrhage |
6 |
(2) |
4 |
(2) |
13 |
(5) |
| Weight Gain |
4 |
(2) |
9 |
(4) |
30 |
(12) |
| Sweating |
4 |
(2) |
3 |
(1) |
16 |
(6) |
| Increased Appetite |
0 |
(0) |
1 |
(0) |
13 |
(5) |
Body as a Whole:
Cardiovascular:
Hepatic:
Hematologic:
Musculoskeletal:
Nervous:
Respiratory:
Skin and Appendages:
Urogenital
| Anastrozole 1 mg (N=262) |
Anastrozole 10 mg (N=246) |
Megestrol Acetate 160 mg (N=253) |
||||
|---|---|---|---|---|---|---|
|
Adverse Reaction Group
|
n
|
(%)
|
n
|
(%)
|
n
|
(%)
|
| Gastrointestinal Disturbance |
77 |
(29) |
81 |
(33) |
54 |
(21) |
| Hot Flushes |
33 |
(13) |
29 |
(12) |
35 |
(14) |
| Edema |
19 |
(7) |
28 |
(11) |
35 |
(14) |
| Thromboembolic Disease |
9 |
(3) |
4 |
(2) |
12 |
(5) |
| Vaginal Dryness |
5 |
(2) |
3 |
(1) |
2 |
(1) |
| Weight Gain |
4 |
(2) |
10 |
(4) |
30 |
(12) |
6.2 Postmarketing Experience
>>
[see Contraindications (4.2)]
7 DRUG INTERACTIONS
7.1 Tamoxifen
[see Clinical Studies (14.1)]
7.2 Estrogen
7.3 Warfarin
max
7.4 Cytochrome P450
in vitroin vivo[see Clinical Pharmacology (12.3)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
[see Contraindications (4.1)]
2 0-24hr2 [see Animal Toxicology and/or Pharmacology (13.2)]
8.3 Nursing Mothers
8.4 Pediatric Use
®
8.5 Geriatric Use
8.6 Renal Impairment
[see Dosage and Administration (2.1) and Clinical Pharmacology (12.3)]
8.7 Hepatic Impairment
[see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)]
10 OVERDOSAGE
11 DESCRIPTION
17195
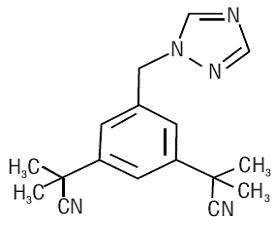
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
Effect on Estradiol
Effect on Corticosteroids
Other Endocrine Effects
12.3 Pharmacokinetics
Absorption
maxmax
Distribution
Metabolism
in vitromaxin vitro
Excretion
Effect of Gender and Age
Effect of Race
Effect of Renal Impairment
2[see Dosage and Administration (2.1) and Use in Specific Populations (8.6)]
Effect of Hepatic Impairment
[see Dosage and Administration (2.2) and Use in Specific Populations (8.7)]
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
2 0-24 hr2
in vitroin vitroin vivo
2 0-24 hr2
ssmax0-24 hrssmax0-24 hr
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology
2 2
ssmax0-24 hr2 2
14 CLINICAL STUDIES
14.1 Adjuvant Treatment of Breast Cancer in Postmenopausal Women
[see Drug Interactions (7.1)]
| Demographic Characteristic | Anastrozole 1 mg (N |
Tamoxifen 20 mg (N |
Anastrozole 1 mg plus Tamoxifen  |
|---|---|---|---|
| Mean age (yrs.) |
64.1 |
64.1 |
64.3 |
| Age Range (yrs.) |
38.1 to 92.8 |
32.8 to 94.9 |
37 to 92.2 |
| Age Distribution (%) |
|||
| <45 yrs. |
0.7 |
0.4 |
0.5 |
| 45 to 60 yrs. |
34.6 |
35 |
34.5 |
| >60 <70 yrs. |
38 |
37.1 |
37.7 |
| >70 yrs. |
26.7 |
27.4 |
27.3 |
| Mean Weight (kg) |
70.8 |
71.1 |
71.3 |
| Receptor Status (%) |
|||
Positive |
83.5 |
83.1 |
84 |
Negative |
7.4 |
8 |
7 |
Other |
8.8 |
8.6 |
9 |
| Other Treatment (%) prior to Randomization |
|||
| Mastectomy |
47.8 |
47.3 |
48.1 |
Breast conservation |
52.3 |
52.8 |
51.9 |
| Axillary surgery |
95.5 |
95.7 |
95.2 |
| Radiotherapy |
63.3 |
62.5 |
61.9 |
| Chemotherapy |
22.3 |
20.8 |
20.8 |
| Neoadjuvant Tamoxifen |
1.6 |
1.6 |
1.7 |
| Primary Tumor Size (%) |
|||
| T1 (≤2 cm) |
63.9 |
62.9 |
64.1 |
| T2 (>2 cm and ≤5 cm) |
32.6 |
34.2 |
32.9 |
| T3 (>5 cm) |
2.7 |
2.2 |
2.3 |
| Nodal Status (%) |
|||
| Node positive |
34.9 |
33.6 |
33.5 |
| 1 to 3 (# of nodes) |
24.4 |
24.4 |
24.3 |
| 4 to 9 |
7.5 |
6.4 |
6.8 |
| >9 |
2.9 |
2.7 |
2.3 |
| Tumor Grade (%) |
|||
| Well-differentiated |
20.8 |
20.5 |
21.2 |
| Moderately differentiated |
46.8 |
47.8 |
46.5 |
| Poorly/undifferentiated |
23.7 |
23.3 |
23.7 |
| Not assessed/recorded |
8.7 |
8.4 |
8.5 |
Intent-To-Treat Population |
Hormone Receptor-Positive Subpopulation |
|||
|---|---|---|---|---|
| Anastrozole 1 mg (N  |
Tamoxifen 20 mg (N  |
Anastrozole 1 mg (N  |
Tamoxifen 20 mg (N  |
|
| Median Duration of Therapy (mo) |
60
|
60
|
60
|
60
|
| Median Efficacy Follow-up (mo) |
68
|
68
|
68
|
68
|
| Loco-regional recurrence |
119 (3.8)
|
149 (4.8)
|
76 (2.9)
|
101 (3.9)
|
| Contralateral breast cancer |
35 (1.1)
|
59 (1.9)
|
26 (1)
|
54 (2.1)
|
| Invasive |
27 (0.9) |
52 (1.7) |
21 (0.8) |
48 (1.8) |
| Ductal carcinoma in situ |
8 (0.3) |
6 (0.2) |
5 (0.2) |
5 (0.2) |
| Unknown |
0 |
1 (<0.1) |
0 |
1 (<0.1) |
| Distant recurrence |
324 (10.4)
|
375 (12)
|
226 (8.6)
|
265 (10.2)
|
| Death from Any Cause |
411 (13.2)
|
420 (13.5)
|
296 (11.3)
|
301 (11.6)
|
| Death breast cancer |
218 (7) |
248 (8) |
138 (5.3) |
160 (6.2) |
| Death other reason (including unknown) |
193 (6.2) |
172 (5.5) |
158 (6) |
141 (5.4) |
| Intent-To-Treat Population | Hormone Receptor-Positive Subpopulation | |||
|---|---|---|---|---|
| Anastrozole 1 mg (N=3125) |
Tamoxifen 20 mg (N=3116) |
Anastrozole 1 mg (N=2618) |
Tamoxifen 20 mg (N=2598) |
|
| Number of Events | Number of Events | |||
|
Disease-free Survival
|
575 |
651 |
424 |
497 |
| Hazard ratio |
0.87 |
0.83 |
||
| 2-sided 95% CI |
0.78 to 0.97 |
0.73 to 0.94 |
||
| p-value |
0.0127 |
0.0049 |
||
|
Distant Disease-free Survival
|
500 |
530 |
370 |
394 |
| Hazard ratio |
0.94 |
0.93 |
||
| 2-sided 95% CI |
0.83 to 1.06 |
0.8 to 1.07 |
||
|
Overall Survival
|
411 |
420 |
296 |
301 |
| Hazard ratio |
0.97 |
0.97 |
||
| 2-sided 95% CI |
0.85 to 1.12 |
0.83 to 1.14 |
||
|
Intent-To-Treat Population
|
Hormone Receptor-
Positive Subpopulation |
|||||
|
Anastrozole
1 mg (N=3125) |
Tamoxifen
20 mg (N=3116) |
Anastrozole
1 mg (N=2618) |
Tamoxifen
20 mg (N=2598) |
|||
|
Number of Events
|
Number of Events
|
|||||
|
Disease-free Survival
|
953 |
1022 |
735 |
924 |
||
| Hazard ratio |
0.91 |
0.86 |
||||
| 2-sided 95% CI |
0.83 to 0.99 |
0.78 to 0.95 |
||||
| p-value |
0.0365 |
0.0027 |
||||
|
Overall Survival
|
734 |
747 |
563 |
586 |
||
| Hazard ratio |
0.97 |
0.95 |
||||
| 2-sided 95% CI |
0.88 to 1.08 |
0.84 to 1.06 |
||||
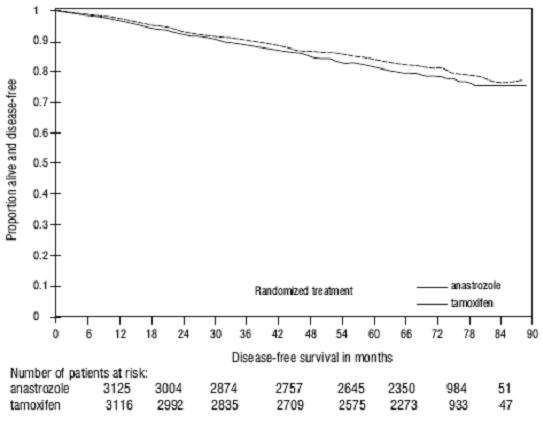
Figure 3 - Disease-Free Survival Kaplan Meier Survival Curve for all Patients Randomized to Anastrozole or Tamoxifen Monotherapy in the ATAC Trial (Intent-to-Treat)(a)
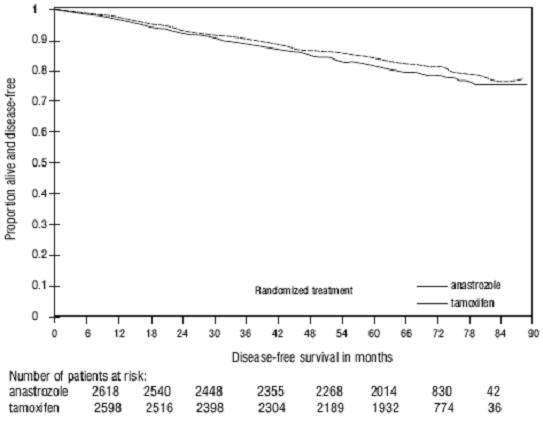
 a
aFigure 4 - Disease-Free Survival for Hormone Receptor-Positive Subpopulation of Patients Randomized to Anastrozole or Tamoxifen Monotherapy in the ATAC Trial(b)
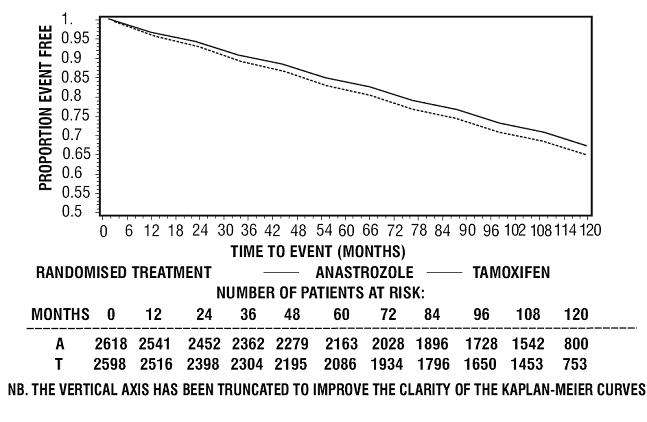 b
b 14.2 First-Line Therapy in Postmenopausal Women with Advanced Breast Cancer
| Number (%) of subjects | ||||
|---|---|---|---|---|
| Trial 0030 | Trial 0027 | |||
| Receptor status | Anastrozole 1 mg (N=171) |
Tamoxifen 20 mg (N=182) |
Anastrozole 1 mg (N=340) |
Tamoxifen 20 mg (N=328) |
ER  |
151 (88.3) |
162 (89) |
154 (45.3) |
144 (43.9) |
| ER* unknown, PgR†
Unknown |
19 (11.1) |
20 (11) |
185 (54.4) |
183 (55.8) |
| Endpoint | Trial 0030 | Trial 0027 | ||
|---|---|---|---|---|
| Anastrozole 1 mg (N=171) |
Tamoxifen 20 mg (N=182) |
Anastrozole 1 mg (N=340) |
Tamoxifen 20 mg (N=328) |
|
| Time to progression (TTP) |
||||
| Median TTP (months) |
11.1 |
5.6 |
8.2 |
8.3 |
| Number (%) of subjects Who progressed |
114 (67%) |
138 (76%) |
249 (73%) |
247 (75%) |
Hazard ratio (LCL  |
1.42 (1.15) |
1.01 (0.87) |
||
2-sided 95% CI |
(1.11, 1.82) |
(0.85, 1.2) |
||
p-value |
0.006 |
0.92 |
||
| Best objective response rate |
||||
Number (%) of subjects With CR  |
36 (21.1%) |
31 (17%) |
112 (32.9%) |
107 (32.6%) |
Odds Ratio (LCL*) |
1.3 (0.83) |
1.01 (0.77) |
||
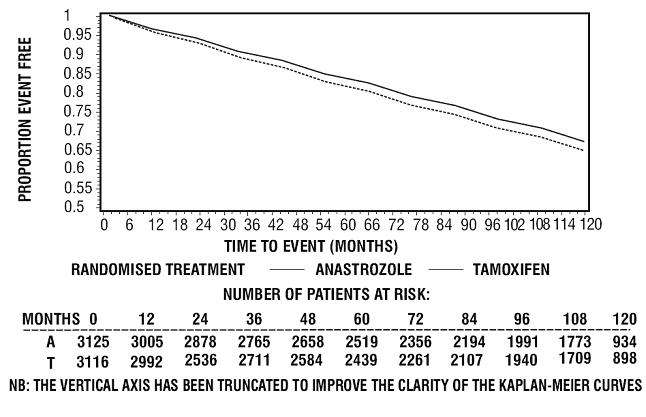
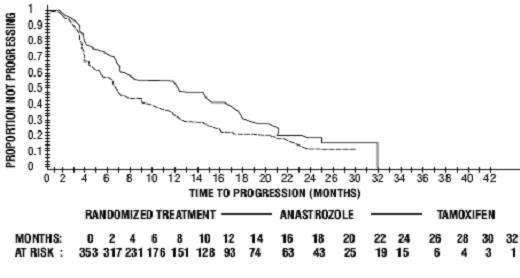 Figure 6 - Kaplan-Meier probability of time to progression for all randomized patients (intent-to-treat) in Trial 0027
Figure 6 - Kaplan-Meier probability of time to progression for all randomized patients (intent-to-treat) in Trial 0027
14.3 Second-Line Therapy in Postmenopausal Women with Advanced Breast Cancer who had Disease Progression following Tamoxifen Therapy
| Anastrozole 1 mg |
Anastrozole 10 mg |
Megestrol Acetate 160 mg |
|
|---|---|---|---|
| Trial 0004 (N. America) |
(N=128) |
(N=130) |
(N=128) |
Median Follow-up (months) |
31.3 |
30.9 |
32.9 |
| Median Time to Death (months) |
29.6 |
25.7 |
26.7 |
| 2 Year Survival Probability (%) |
62 |
58 |
53.1 |
| Median Time to Progression (months) |
5.7 |
5.3 |
5.1 |
| Objective Response (all patients) (%) |
12.5 |
10 |
10.2 |
| Stable Disease for >24 weeks (%) |
35.2 |
29.2 |
32.8 |
| Progression (%) |
86.7 |
85.4 |
90.6 |
| Trial 0005 (Europe, Australia, S. Africa) |
(N=135) |
(N=118) |
(N=125) |
| Median Follow-up (months)* |
31 |
30.9 |
31.5 |
| Median Time to Death (months) |
24.3 |
24.8 |
19.8 |
| 2 Year Survival Probability (%) |
50.5 |
50.9 |
39.1 |
| Median Time to Progression (months) |
4.4 |
5.3 |
3.9 |
| Objective Response (all patients) (%) |
12.6 |
15.3 |
14.4 |
| Stable Disease for >24 weeks (%) |
24.4 |
25.4 |
23.2 |
| Progression (%) |
91.9 |
89.8 |
92 |
| Trials 0004 & 0005 (Pooled Data) |
Anastrozole 1 mg N=263 |
Anastrozole 10 mg N=248 |
Megestrol Acetate 160 mg N=253 |
|---|---|---|---|
| Median Time to Death (months) |
26.7 |
25.5 |
22.5 |
| 2 Year Survival Probability (%) |
56.1 |
54.6 |
46.3 |
| Median Time to Progression |
4.8 |
5.3 |
4.6 |
| Objective Response (all patients) (%) |
12.5 |
12.5 |
12.3 |
16 HOW SUPPLIED/STORAGE AND HANDLING
Storage
17 PATIENT COUNSELING INFORMATION
17.1 Pregnancy
17.2 Allergic (Hypersensitivity) Reactions
17.3 Ischemic Cardiovascular Events
17.4 Bone Effects
17.5 Cholesterol
17.6 Tamoxifen
FDA-Approved Patient Labeling
PATIENT INFORMATION
Anastrozole Tablets
What is anastrozole?
- treatment of early breast cancer
- after surgery, with or without radiation
- in women whose breast cancer is hormone receptor-positive
- first treatment of locally advanced or metastatic breast cancer, in women whose breast cancer is hormone receptor-positive or the hormone receptors are not known.
- treatment of advanced breast cancer, if the cancer has grown, or the disease has spread after tamoxifen therapy.
Who should not take anastrozole tablet?
- are pregnant, think you may be pregnant, or plan to get pregnant. Anastrozole may harm your unborn child. If you become pregnant while taking anastrozole, tell your doctor right away.
- have not finished menopause (are premenopausal)
- are allergic to any of the ingredients in anastrozole tablets. See the end of this leaflet for a list of the ingredients in anastrozole tablets.
- are a man or child
-
Heart disease. Women with early breast cancer, who have a history of blockages in heart arteries (ischemic heart disease) and who take anastrozole may have a slight increase in this type of heart disease compared to similar patients who take tamoxifen.
- Stop taking anastrozole and call your doctor right away if you have chest pain or shortness of breath. These can be symptoms of heart disease.
- Osteoporosis (bone softening and weakening). Anastrozole lowers estrogen in your body, which may cause your bones to become softer and weaker. This can increase your chance of fractures, specifically of the spine, hip and wrist. Your doctor may order a test for you called a bone mineral density study before you start taking anastrozole and during treatment with anastrozole as needed.
Anastrozole may not be right for you. Before taking anastrozole, tell your doctor about all your medical conditions, including if you:
- have not finished menopause. Talk to your doctor if you are not sure. See “Who should not take anastrozole tablets?”
- have had a previous heart problem
- have a condition called osteoporosis
- have high cholesterol
- are pregnant, planning to become pregnant, or breast feeding. See “Who should not take anastrozole tablets?”
- are nursing a baby. It is not known if anastrozole passes into breast milk. You and your doctor should decide if you will take anastrozole tablets or breast feed. You should not do both.
- Tamoxifen. You should not take anastrozole with tamoxifen. Taking tamoxifen with anastrozole may lower the amount of anastrozole in your blood and may cause anastrozole not to work as well.
-
Medicines containing estrogen. Anastrozole may not work if taken with one of these medicines:
- hormone replacement therapy
- birth control pills
- estrogen creams
- vaginal rings
- vaginal suppositories
How should I take anastrozole tablets?
- Take anastrozole tablets exactly as prescribed by your doctor. Keep taking anastrozole tablets for as along as your doctor prescribes it for you.
- Take one anastrozole tablet each day.
- Anastrozole can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Take your next regularly scheduled dose. Do not take two doses at the same time.
- If you have taken more anastrozole tablets than your doctor has prescribed, contact your doctor right away. Do not take any additional anastrozole tablets until instructed to do so by your doctor.
What are possible side effects of anastrozole?
- See “What is the most important information I should know about anastrozole?”
- increased blood cholesterol (fat in the blood). Your doctor may check your cholesterol while you take anastrozole therapy.
- skin reactions. Stop taking anastrozole and call your doctor right away if you get any skin lesions, ulcers, or blisters.
-
severe allergic reactions. Get medical help right away if you have:
- swelling of the face, lips, tongue, or throat.
- trouble swallowing
- trouble breathing
-
liver problems. Anastrozole can cause inflammation of the liver and changes in blood tests of the liver function. Your doctor may monitor you for this. Stop taking anastrozole and call your doctor right away if you have any of these signs or symptoms of a liver problem:
- a general feeling of not being well
- yellowing of the skin or whites of the eyes
- pain on the right side of your abdomen
- hot flashes
- weakness
- joint pain
- carpal tunnel syndrome (tingling, pain, coldness, weakness in parts of the hand)
- pain
- sore throat
- mood changes
- high blood pressure
- depression
- nausea and vomiting
- thinning of the hair (hair loss)
- rash
- back pain
- sleep problems
- bone pain
- headache
- swelling
- increased cough
- shortness of breath
- lymphedema (build up of lymph fluid in the tissues of your affected arm)
- trigger finger (a condition in which one of your fingers or your thumb catches in a bent position)
HOW SHOULD I STORE ANASTROZOLE TABLETS?
- Store anastrozole tablets at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F).
- Keep anastrozole and all medicines out of the reach of children.
What are the ingredients in anastrozole tablets?
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 1MG
NDC 62756-250-88
Anastrozole Tablets
1 mg
Rx only
100 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
Pharmacist: Dispense patient leaflet to each patient.

ANASTROZOLEANASTROZOLE TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||