ANASTROZOLE
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use anastrozole tablets safely and effectively. See full prescribing information for anastrozole tablets. Initial U.S. ApprovalAnastrozole tablets for oral use RECENT MAJOR CHANGESContraindications - Premenopausal Women and Pregnancy(4.1, 8.1) 01/2010 Warnings and Precautions- Ischemic Cardiovascular Events (5.1, 6.1) 01/2010INDICATIONS AND USAGE Adjuvant treatment of postmenopausal women with hormone receptor-positive early breast cancer (1.1) First-line treatment of postmenopausal women with hormone receptor-positive or hormone receptor unknown locally advanced or metastatic breast cancer (1.2) Treatment of advanced breast cancer in postmenopausal women with disease progression following tamoxifen therapy. Patients with ER-negative disease and patients who did not respond to previous tamoxifen therapy rarely responded to anastrozole tablets (1.3) DOSAGE AND ADMINISTRATIONOne 1 mg tablet taken once daily (2.1) DOSAGE FORMS AND STRENGTHS1 mg tablets (3) CONTRAINDICATIONS Women of premenopausal endocrine status, including pregnant women (4.1, 8.1) Patients with demonstrated hypersensitivity to anastrozole tablets or any excipient (4.2) WARNINGS AND PRECAUTIONS In women with pre-existing ischemic heart disease, an increased incidence of ischemic cardiovascular events occurred with anastrozole tablet use compared to tamoxifen use. Consider risks and benefits. (5.1, 6.1) Decreases in bone mineral density may occur. Consider bone mineral density monitoring. (5.2, 6.1) Increases in total cholesterol may occur. Consider cholesterol monitoring. (5.3, 6.1) Side EffectsTo report suspected adverse reactions and 1-800-FDA-1088 or .DRUG INTERACTIONS Tamoxifen: Do not use in combination with anastrozole tablets. No additional benefit seen over tamoxifen monotherapy (7.1, 14.1). Estrogen-containing products: Combination use may diminish activity of anastrozole tablets (7.2). USE IN SPECIFIC POPULATIONS Pediatric patients: Efficacy has not been demonstrated for pubertal boys of adolescent age with gynecomastia or girls with McCune-Albright Syndrome and progressive precocious puberty. (8.4)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ANASTROZOLE INDICATIONS AND USAGE
- 2 ANASTROZOLE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ANASTROZOLE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ANASTROZOLE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 ANASTROZOLE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- Anastrozole tablets 1mg - 30 tablets in a Box-Unit Dose
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Adjuvant Treatment
1.2 First-Line Treatment
Anastrozole tablets are indicated for the first-line treatment of postmenopausal women with hormone receptor-positive or hormone receptor unknown locally advanced or metastatic breast cancer.
1.3 Second-Line Treatment
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
[see Clinical Studies (14.1)]
[see Use in Specific Populations (8.6)]
2.2 Patients with Hepatic Impairment
No changes in dose are recommended for patients with mild-to-moderate hepatic impairment. Anastrozole tablets have not been studied in patients with severe hepatic impairment. [see Use in Specific Populations (8.7)]
3 DOSAGE FORMS AND STRENGTHS
The tablets are white, biconvex, film-coated containing 1 mg of anastrozole. The tablets are debossed with “AN” and “1” on one side and plain surface on the other side.
4 CONTRAINDICATIONS
4.1 Pregnancy and Premenopausal Women
Anastrozole tablets may cause fetal harm when administered to a pregnant woman and offers no clinical benefit to premenopausal women with breast cancer. Anastrozole tablets are contraindicated in women who are or may become pregnant. There are no adequate and well-controlled studies in pregnant women using anastrozole tablets. If anastrozole tablets are used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus or potential risk for loss of the pregnancy. [see Use in Specific Populations (8.1)]
4.2 Hypersensitivity
Anastrozole tablets are contraindicated in any patient who has shown a hypersensitivity reaction to the drug or to any of the excipients. Observed reactions include anaphylaxis, angioedema, and urticaria. [see Adverse Reactions (6.2)]
5 WARNINGS AND PRECAUTIONS
5.1 Ischemic Cardiovascular Events
In women with pre-existing ischemic heart disease, an increased incidence of ischemic cardiovascular events was observed with anastrozole tablets in the ATAC trial (17% of patients on anastrozole tablets and 10% of patients on tamoxifen). Consider risk and benefits of anastrozole tablets therapy in patients with pre-existing ischemic heart disease. [see Adverse Reactions (6.1)]
5.2 Bone Effects
Results from the ATAC trial bone substudy at 12 and 24 months demonstrated that patients receiving anastrozole tablets had a mean decrease in both lumbar spine and total hip bone mineral density (BMD) compared to baseline. Patients receiving tamoxifen had a mean increase in both lumbar spine and total hip BMD compared to baseline [see Adverse Reactions, (6.1)].
5.3 Cholesterol
During the ATAC trial, more patients receiving anastrozole tablets were reported to have elevated serum cholesterol compared to patients receiving tamoxifen (9% versus 3.5%, respectively) [see Adverse Reactions, (6.1)].
6 ADVERSE REACTIONS
[see Adverse Reactions, (6.2)].
6.1 Clinical Trials Experience
Adjuvant Therapy
[see Clinical Studies (14.1) ].
Table 1 - Adverse reactions occurring with an incidence of at least 5% in either treatment group during treatment, or within 14 days of the end of
treatment in the ATAC trial*
|
* The combination arm was discontinued due to lack of efficacy benefit at 33 months of follow-up. † COSTART Coding Symbols for Thesaurus of Adverse Reaction Terms. ‡ A patient may have had more than 1 adverse reaction, including more than 1 adverse reaction in the same body system. § N=Number of patients receiving the treatment. ¶ Vaginal Hemorrhage without further diagnosis. |
||
|
Body system and adverse reactions by COSTART† preferred term
|
Anastrozole tablets 1mg
(N§ = 3092) |
Tamoxifen 20 mg
(N§ = 3094) |
|
Body as a whole
|
|
|
| Asthenia |
575 (19) |
544 (18) |
| Pain |
533 (17) |
485 (16) |
| Back pain |
321 (10) |
309 (10) |
| Headache |
314 (10) |
249 (8) |
| Abdominal pain |
271 (9) |
276 (9) |
| Infection |
285 (9) |
276 (9) |
| Accidental injury |
311 (10) |
303 (10) |
| Flu syndrome |
175 (6) |
195 (6) |
| Chest pain |
200 (7) |
150 (5) |
| Neoplasm |
162 (5) |
144 (5) |
| Cyst |
138 (5) |
162 (5) |
|
Cardiovascular
|
||
| Vasodilatation
|
1104 (36) |
1264 (41) |
| Hypertension
|
402 (13) |
349 (11) |
|
Digestive
|
|
|
| Nausea
|
343 (11) |
335 (11) |
| Constipation
|
249 (8) |
252 (8) |
| Diarrhea |
265 (9) |
216 (7) |
| Dyspepsia |
206 (7) |
169 (6) |
| Gastrointestinal disorder |
210 (7) |
158 (5) |
|
Hemic and lymphatic
|
||
| Lymphedema |
304 (10) |
341 (11) |
| Anemia |
113 (4) |
159 (5) |
|
Metabolic and nutritional
|
||
| Peripheral edema |
311 (10) |
343 (11) |
| Weight gain |
285 (9) |
274 (9) |
| Hypercholesterolemia |
278 (9) |
108 (3.5) |
|
Musculoskeletal
|
||
| Arthritis |
512 (17) |
445 (14) |
| Arthralgia |
467 (15) |
344 (11) |
| Osteoporosis |
325 (11) |
226 (7) |
| Fracture |
315 (10) |
209 (7) |
| Bone pain |
201 (7) |
185 (6) |
| Arthrosis |
207 (7) |
156 (5) |
| Joint Disorder |
184 (6) |
160 (5) |
| Myalgia |
179 (6) |
160 (5) |
|
Nervous system
|
||
| Depression |
413 (13) |
382 (12) |
| Insomnia |
309 (10) |
281 (9) |
| Dizziness |
236 (8) |
234 (8)
|
| Anxiety |
195 (6) |
180 (6) |
| Paresthesia |
215 (7) |
145 (5) |
|
Respiratory
|
||
| Pharyngitis |
443 (14) |
422 (14) |
| Cough increased |
261 (8) |
287 (9) |
| Dyspnea |
234 (8) |
237 (8) |
| Sinusitis |
184 (6) |
159 (5) |
| Bronchitis |
167 (5) |
153 (5) |
|
Skin and appendages
|
||
| Rash |
333 (11) |
387 (13) |
| Sweating |
145 (5) |
177 (6) |
|
Special Senses
|
||
| Cataract Specified |
182 (6) |
213 (7) |
|
Urogenital
|
||
| Leukorrhea |
86 (3) |
286 (9) |
| Urinary tract infection |
244 (8) |
313 (10) |
| Breast pain |
251 (8) |
169 (6) |
| Breast Neoplasm |
164 (5) |
139 (5) |
| Vulvovaginitis |
194 (6) |
150 (5) |
| Vaginal Hemorrhage¶
|
122 (4) |
180 (6) |
| Vaginitis |
125 (4) |
158 (5) |
Table 2 — Number of Patients with Pre-specified Adverse Reactions in ATAC Trial*
|
* Patients with multiple events in the same category are counted only once in that category. † Refers to joint symptoms, including joint disorder, arthritis, arthrosis and arthralgia. ‡ Percentages calculated based upon the numbers of patients with an intact uterus at Baseline |
||||
|
|
Anastrozole tablets
N=3092 (%) |
Tamoxifen
N=3094 (%) |
Odds-ratio
|
95% CI
|
| Hot Flashes
|
1104 (36)
|
1264(41)
|
0.80
|
0.73 - 0.89
|
| Musculoskeletal Events†
Fatigue/Asthenia |
1100 (36) 575 (19) |
911 (29) 544 (18) |
1.32 1.07 |
1.19 - 1.47 0.94 - 1.22 |
| Mood Disturbances
|
597 (19)
|
554 (18)
|
1.10
|
0.97 - 1.25
|
| Nausea and Vomiting
|
393 (13)
|
384 (12)
|
1.03
|
0.88 - 1.19
|
| All Fractures
|
315 (10)
|
209 (7)
|
1.57
|
1.30 - 1.88
|
| Fractures of Spine, Hip, or Wrist |
133 (4)
|
91 (3)
|
1.48
|
1.13 - 1.95
|
| Wrist/Colles’ fractures |
67 (2) |
50 (2) |
|
|
| Spine fractures |
43 (1) |
22 (1) |
|
|
| Hip fractures |
28 (1) |
26 (1) |
|
|
| Cataracts |
182 (6) |
213 (7) |
0.85 |
0.69 - 1.04 |
| Vaginal Bleeding |
167 (5) |
317 (10) |
0.50 |
0.41 - 0.61 |
| Ischemic Cardiovascular Disease |
127 (4) |
104 (3) |
1.23 |
0.95 - 1.60 |
| Vaginal Discharge |
109 (4) |
408 (13) |
0.24 |
0.19 - 0.30 |
| Venous Thromboembolic events |
87 (3) |
140 (5) |
0.61 |
0.47 - 0.80 |
| Deep Venous Thromboembolic Events |
48 (2) |
74 (2) |
0.64 |
0.45 - 0.93 |
| Ischemic Cerebrovascular Event |
62 (2) |
88 (3) |
0.70 |
0.50 - 0.97 |
| Endometrial Cancer‡
|
4 (0.2) |
13 (0.6) |
0.31 |
0.10 - 0.94 |
Ischemic Cardiovascular Events
In women with pre-existing ischemic heart disease 465/6186 (7.5%), the incidence of ischemic cardiovascular events was 17% in patients on anastrozole tablets and 10% in patients on tamoxifen. In this patient population, angina pectoris was reported in 25/216 (11.6%) patients receiving anastrozole tablets and 13/249 (5.2%) patients receiving tamoxifen; myocardial infarction was reported in 2/216 (0.9%) patients receiving anastrozole tablets and 8/249 (3.2%) patients receiving tamoxifen.
Bone Mineral Density Findings
Results from the ATAC trial bone substudy at 12 and 24 months demonstrated that patients receiving anastrozole tablets had a mean decrease in both lumbar spine and total hip bone mineral density (BMD) compared to baseline. Patients receiving tamoxifen had a mean increase in both lumbar spine and total hip BMD compared to baseline.
Cholesterol
Other Adverse Reactions
First-Line Therapy
Table 3 – Adverse Reactions Occurring with an Incidence of at Least 5% in Trials 0030 and 0027
|
* A patient may have had more than 1 adverse event. |
||
|
Body system
Adverse Reaction* |
Number (%) of subjects
|
|
|
Anastrozole tablets
(N=506) |
Tamoxifen
(N=511) |
|
|
Whole body
|
||
| Asthenia
|
83 (16)
|
81 (16)
|
| Pain
|
70 (14)
|
73 (14)
|
| Back pain |
60 (12) |
68 (13) |
| Headache |
47 (9) |
40 (8) |
| Abdominal pain |
40 (8) |
38 (7) |
| Chest pain |
37 (7) |
37 (7) |
| Flu syndrome |
35 (7) |
30 (6) |
| Pelvic pain |
23 (5) |
30 (6) |
|
Cardiovascular
|
|
|
| Vasodilation |
128 (25) |
106 (21) |
| Hypertension |
25 (5) |
36 (7) |
|
Digestive
|
||
| Nausea |
94 (19) |
106 (21) |
| Constipation |
47 (9) |
66 (13) |
| Diarrhea |
40 (8) |
33 (6) |
| Vomiting |
38 (8) |
36 (7) |
| Anorexia |
26 (5) |
46 (9) |
|
Metabolic and Nutritional
|
||
| Peripheral edema |
51 (10) |
41 (8) |
|
Muscoloskeletal
|
||
| Bone pain |
54 (11) |
52 (10) |
|
Nervous
|
||
| Dizziness
|
30 (6) |
22 (4) |
| Insomnia
|
30 (6) |
38 (7) |
| Depression
|
23 (5) |
32 (6) |
| Hypertonia
|
16 (3) |
26 (5) |
|
Respiratory
|
||
| Cough increased
|
55 (11) |
52 (10) |
| Dyspnea
|
51 (10) |
47 (9) |
| Pharyngitis
|
49 (10) |
68 (13) |
|
Skin and appendages
|
||
| Rash
|
38 (8) |
34 (8) |
|
Urogenital
|
||
| Leukorrhea
|
9 (2) |
31 (6) |
Table 4 – Number of Patients with Pre-specified Adverse Reactions in Trials 0030 and 0027
|
* A patient may have had more than 1 adverse event. † Includes pulmonary embolus, thrombophlebitis, retinal vein thrombosis. ‡ Includes myocardial infarction, myocardial ischemia, angina pectoris, cerebrovascular accident, cerebral ischemia and cerebral infarct. |
||
|
Number (n) and Percentage of Patients
|
||
|
Adverse Reaction*
|
Anastrozole tablets 1 mg
(N=506) n (%) |
NOLVADEX 20 mg
(N=511) n (%) |
| Depression
|
23 (5)
|
32 (6)
|
| Tumor Flare
|
15 (3)
|
18 (4)
|
| Thromboembolic Disease†
|
18 (4)
|
33 (6)
|
| Venous†
|
5
|
15
|
| Coronary and Cerebral‡
|
13
|
19 |
| Gastrointestinal Disturbance |
170 (34) |
196 (38) |
| Hot Flushes |
134 (26) |
118 (23) |
| Vaginal Dryness |
9 (2) |
3 (1) |
| Lethargy |
6 (1) |
15 (3) |
| Vaginal Bleeding |
5 (1) |
11 (2) |
| Weight Gain |
11 (2) |
8 (2) |
Second-Line Therapy
Anastrozole tablets were tolerated in two controlled clinical trials (i.e., Trials 0004 and 0005), with less than 3.3% of the anastrozole tablets-treated patients and 4.0% of the megestrol acetate-treated patients withdrawing due to an adverse reaction.
The principal adverse reaction more common with anastrozole tablets than megestrol acetate was diarrhea. Adverse reactions reported in greater than 5% of the patients in any of the treatment groups in these two controlled clinical trials, regardless of causality, are presented below:
Table 5 - Number (N) and Percentage of Patients with Adverse Reactions in Trials 0004 and 0005
|
* A patient may have had more than one adverse reaction. |
||||||
|
Adverse Reaction*
|
Anastrozole tablets 1 mg
|
Anastrozole tablets 10 mg
|
Megestrol Acetate 160 mg
|
|||
|
(N=262)
|
(N=246)
|
(N=253)
|
||||
|
n
|
%
|
n
|
%
|
n
|
%
|
|
| Asthenia
|
42
|
(16)
|
33
|
(13)
|
47
|
(19)
|
| Nausea
|
41
|
(16)
|
48
|
(20)
|
28
|
(11)
|
| Headache
|
34
|
(13)
|
44
|
(18)
|
24
|
(9)
|
| Hot Flashes
|
32
|
(12)
|
29
|
(11)
|
21
|
(8)
|
| Pain
|
28
|
(11)
|
38
|
(15)
|
29
|
(11)
|
| Back Pain
|
28
|
(11)
|
26
|
(11)
|
19
|
(8)
|
| Dyspnea
|
24
|
(9)
|
27
|
(11)
|
53
|
(21)
|
| Vomiting |
24 |
(9) |
26 |
(11) |
16 |
(6) |
| Cough Increased |
22 |
(8) |
18 |
(7) |
19 |
(8) |
| Diarrhea |
22 |
(8) |
18 |
(7) |
7 |
(3) |
| Constipation |
18 |
(7) |
18 |
(7) |
21 |
(8) |
| Abdominal Pain |
18 |
(7) |
14 |
(6) |
18 |
(7) |
| Anorexia |
18 |
(7) |
19 |
(8) |
11 |
(4) |
| Bone Pain |
17 |
(6) |
26 |
(12) |
19 |
(8) |
| Pharyngitis |
16 |
(6) |
23 |
(9) |
15 |
(6) |
| Dizziness |
16 |
(6) |
12 |
(5) |
15 |
(6) |
| Rash |
15 |
(6) |
15 |
(6) |
19 |
(8) |
| Dry Mouth |
15 |
(6) |
11 |
(4) |
13 |
(5) |
| Peripheral Edema |
14 |
(5) |
21 |
(9) |
28 |
(11) |
| Pelvic Pain |
14 |
(5) |
17 |
(7) |
13 |
(5) |
| Depression |
14 |
(5) |
6 |
(2) |
5 |
(2) |
| Chest Pain |
13 |
(5) |
18 |
(7) |
13 |
(5) |
| Paresthesia |
12 |
(5) |
15 |
(6) |
9 |
(4) |
| Vaginal Hemorrhage |
6 |
(2) |
4 |
(2) |
13 |
(5) |
| Weight Gain |
4 |
(2) |
9 |
(4) |
30 |
(12) |
| Sweating |
4 |
(2) |
3 |
(1) |
16 |
(6) |
| Increased Appetite |
0 |
(0) |
1 |
(0) |
13 |
(5) |
Body as a Whole
Cardiovascular: Hypertension; thrombophlebitis
Hepatic:
Hematologic:
Metabolic and Nutritional: Alkaline phosphatase increased; weight loss
Mean serum total cholesterol levels increased by 0.5 mmol/L among patients receiving anastrozole tablets. Increases in LDL cholesterol have been shown to contribute to these changes.
Musculoskeletal: Myalgia; arthralgia; pathological fracture
Nervous: Somnolence; confusion; insomnia; anxiety; nervousness
Respiratory: Sinusitis; bronchitis; rhinitis
Skin and Appendages: Hair thinning (alopecia); pruritus
Urogenital:
Table 6 — Number (n) and Percentage of Patients with Pre-specified Adverse Reactions in Trials 0004 and 0005
|
|
Anastrozole tablets 1 mg
|
Anastrozole tablets 10 mg
|
Megestrol Acetate 160 mg
|
|||
|
(N=262)
|
(N=246)
|
(N=253)
|
||||
|
Adverse Event
Group |
n
|
(%)
|
n
|
(%)
|
n
|
(%)
|
| Gastrointestinal Disturbance |
77
|
(29)
|
81
|
(33)
|
54
|
(21)
|
| Hot Flushes
|
33
|
(13)
|
29
|
(12)
|
35
|
(14)
|
| Edema
|
19
|
(7)
|
28
|
(11)
|
35
|
(14)
|
| Thromboembolic Disease |
9
|
(3)
|
4
|
(2)
|
12
|
(5)
|
| Vaginal Dryness |
5 |
(2) |
3 |
(1) |
2 |
(1) |
| Weight Gain |
4 |
(2) |
10 |
(4) |
30 |
(12) |
6.2 Post-Marketing Experience
Anastrozole tablets may also be associated with rash including cases of mucocutaneous disorders such as erythema multiforme and Stevens-Johnson syndrome.
Cases of allergic reactions including angioedema, urticaria and anaphylaxis have been reported in patients receiving anastrozole tablets. [see Contraindications (4.2)]
Trigger finger has been reported (≥0.1% and <1%) in patients receiving anastrozole tablets.
7 DRUG INTERACTIONS
7.1 Tamoxifen
Co-administration of anastrozole and tamoxifen in breast cancer patients reduced anastrozole plasma concentration by 27%. However, the coadministration of anastrozole and tamoxifen did not affect the pharmacokinetics of tamoxifen or N-desmethyltamoxifen. At a median follow-up of 33 months, the combination of anastrozole tablets and tamoxifen did not demonstrate any efficacy benefit when compared with tamoxifen in all patients as well as in the hormone receptor-positive subpopulation. This treatment arm was discontinued from the trial. [see Clinical Studies (14.1)]. Based on clinical and pharmacokinetic results from the ATAC trial, tamoxifen should not be administered with anastrozole.
7.2 Estrogen
Estrogen-containing therapies should not be used with anastrozole tablets as they may diminish its pharmacological action.
7.3 Warfarin
In a study conducted in 16 male volunteers, anastrozole did not alter the exposure (as measured by Cmax and AUC) and anticoagulant activity (as measured by prothrombin time, activated partial thromboplastin time, and thrombin time) of both R- and S-warfarin.
7.4 Cytochrome P450
Based on in vitro and in vivo results, it is unlikely that co-administration of anastrozole tablets 1 mg will affect other drugs as a result inhibition of cytochrome P450 [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
[see Contraindications (4.1)]
20-24hr 2[see Animal Toxicology and/or Pharmacology (13.2)]
8.2 Labor & Delivery
8.3 Nursing Mothers
It is not known if anastrozole is excreted in human milk. Because many drugs are excreted in human milk and because of the tumorigenicity shown for anastrozole in animal studies, or the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The efficacy of anastrozole tablets in the treatment of pubertal gynecomastia in adolescent boys and in the treatment of precocious puberty in girls with McCune-Albright Syndrome has not been demonstrated.
Labeling describing clinical trails and pharmacokinetic studies of anastrozole in pubertal boys of adolescent age with gynecomastia and in girls with McCune- Albright Syndrome and progressive precocious puberty is approved for AstraZeneca Pharmaceuticals LP’s Arimidex ® . However, due to AstraZeneca Pharmaceuticals LP’s marketing exclusivity rights, a description of those trials and studies is not approved for this anastrozole labeling.
8.5 Geriatric Use
The pharmacokinetics of anastrozole are not affected by age.
8.6 Renal Impairment
Since only about 10% of anastrozole is excreted unchanged in the urine, the renal impairment does not influence the total body clearance. Dosage adjustment in patients with renal impairment is not necessary [see Dosage and Administration (2.1) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
The plasma anastrozole concentrations in the subjects with hepatic cirrhosis were within the range of concentrations seen in normal subjects across all clinical trials. Therefore, dosage adjustment is also not necessary in patients with stable hepatic cirrhosis. Anastrozole tablets have not been studied in patients with severe hepatic impairment [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Clinical trials have been conducted with anastrozole tablets, up to 60 mg in a single dose given to healthy male volunteers and up to 10 mg daily given to postmenopausal women with advanced breast cancer; these dosages were tolerated. A single dose of anastrozole tablets that results in life-threatening symptoms has not been established. There is no specific antidote to overdosage and treatment must be symptomatic. In the management of an overdose, consider that multiple agents may have been taken. Vomiting may be induced if the patient is alert. Dialysis may be helpful because anastrozole tablets are not highly protein bound. General supportive care, including frequent monitoring of vital signs and close observation of the patient, is indicated.
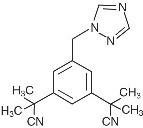
11 DESCRIPTION
17195
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Anastrozole is a potent and selective non-steroidal aromatase inhibitor. It significantly lowers serum estradiol concentrations and has no detectable effect on formation of adrenal corticosteroids or aldosterone.
12.2 Pharmacodynamics
Effect on Estradiol
Effect on Corticosteroids
Other Endocrine Effects
12.3 Pharmacokinetics
Absorption
maxmax
Distribution
Metabolism
in vitro maxin vitro
Excretion
Effect of Gender and Age
Effect of Race
Effect of Renal Impairment
2 [see Dosage and Administration (2.1) and Use in Specific Populations (8.6)]
Effect of Hepatic Impairment
[see Dosage and Administration (2.2) and Use in Specific Populations (8.7)]
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
20-24 hr 2
in vitroin vitroin vivo
20-24 hr2
Multiple-dose studies in rats administered anastrozole for 6 months at doses equal to or greater than 1 mg/kg/day (which produced plasma anastrozole Cssmax and AUC0-24 hr that were 19 and 9 times higher than the respective values found in postmenopausal volunteers at the recommended dose) resulted in hypertrophy of the ovaries and the presence of follicular cysts. In addition, hyperplastic uteri were observed in 6-month studies in female dogs administered doses equal to or greater than 1 mg/kg/day (which produced plasma anastrozole Cssmax and AUC0-24 hr that were 22 times and 16 times higher than the respective values found in postmenopausal women at the recommended dose). It is not known whether these effects on the reproductive organs of animals are associated with impaired fertility in premenopausal women.
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology
22
Evidence of fetotoxicity, including delayed fetal development (i.e., incomplete ossification and depressed fetal body weights), was observed in rats administered doses of 1 mg/kg/day (which produced plasma anastrozole Cssmax and AUC0-24 hr that were 19 times and 9 times higher than the respective values found in postmenopausal volunteers at the recommended dose). There was no evidence of teratogenicity in rats administered doses up to 1.0 mg/kg/day. In rabbits, anastrozole caused pregnancy failure at doses equal to or greater than 1.0 mg/kg/day (about 16 times the recommended human dose on a mg/m2 basis); there was no evidence of teratogenicity in rabbits administered 0.2 mg/kg/day (about 3 times the recommended human dose on a mg/m2 basis).
14 CLINICAL STUDIES
14.1 Adjuvant Treatment of Breast Cancer in Postmenopausal Women
A multicenter, double-blind trial (ATAC) randomized 9,366 postmenopausal women with operable breast cancer to adjuvant treatment with anastrozole tablets 1 mg daily, tamoxifen 20 mg daily, or a combination of the two treatments for five years or until recurrence of the disease.
The primary endpoint of the trial was disease-free survival (i.e., time to occurrence of a distant or local recurrence, or contralateral breast cancer or death from any cause). Secondary endpoints of the trial included distant disease-free survival, the incidence of contralateral breast cancer and overall survival. At a median follow-up of 33 months, the combination of anastrozole tablets and tamoxifen did not demonstrate any efficacy benefit when compared with tamoxifen in all patients as well as in the hormone receptor positive subpopulation. This treatment arm was discontinued from the trial. Based on clinical and pharmacokinetic results from the ATAC trial, tamoxifen should not be administered with anastrozole. [see Drug Interactions (7.1)]
Demographic and other baseline characteristics were similar among the three treatment groups (see Table 7).
Table 7 - Demographic and Baseline Characteristics for ATAC Trial
|
* N=Number of patients randomized to the treatment † The combination arm was discontinued due to lack of efficacy benefit at 33 months of follow-up ‡ Includes patients who were estrogen receptor (ER) positive or progesterone receptor (PgR) positive, or both positive § Includes patients with both ER negative and PgR negative receptor status ¶Includes all other combinations of ER and PgR receptor status unknown #Among the patients who had breast conservation, radiotherapy was administered to 95.0% of patients in the anastrozole tablets arm, 94.1% in the tamoxifen arm and 94.5% in the anastrozole tablets plus tamoxifen arm. |
|||
|
Demographic Characteristic |
Anastrozole tablets 1 mg |
Tamoxifen 20 mg |
Anastrozole tablets 1 mg plus Tamoxifen 20 mg |
|
(N*=3125) |
(N*=3116) |
(N*=3125) |
|
|
Mean age (yrs.) |
64.1 |
64.1 |
64.3 |
|
AgeRange (yrs.) |
38.1 - 92.8 |
32.8 - 94.9 |
37.0 – 92.2 |
|
Age Distribution (%) |
|||
|
<45 yrs. |
0.7 |
0.4 |
0.5 |
|
45-60 yrs. |
34.6 |
35.0 |
34.5 |
|
>60 <70 yrs. |
38.0 |
37.1 |
37.7 |
|
>70 yrs. |
26.7 |
27.4 |
27.3 |
|
Mean Weight (kg) |
70.8 |
71.1 |
71.3 |
|
Receptor Status(%) |
|||
|
Positive‡ |
83.5 |
83.1 |
84.0 |
|
Negative§ |
7.4 |
8.0 |
7.0 |
|
Other¶ |
8.8 |
8.6 |
9.0 |
|
Other Treatment (%) prior to Randomization |
|||
|
Mastectomy |
47.8 |
47.3 |
48.1 |
|
Breast conservation# |
52.3 |
52.8 |
51.9 |
|
Axillary surgery |
95.5 |
95.7 |
95.2 |
|
Radiotherapy |
63.3 |
62.5 |
61.9 |
|
Chemotherapy |
22.3 |
20.8 |
20.8 |
|
Neoadjuvant Tamoxifen |
1.6 |
1.6 |
1.7 |
|
Primary Tumor Size (%) |
|||
|
T1 (≤2 cm) |
63.9 |
62.9 |
64.1 |
|
T2 (>2 cm and ≤5 cm) |
32.6 |
34.2 |
32.9 |
|
T3 (>5 cm) |
2.7 |
2.2 |
2.3 |
|
Nodal Status (%) |
|||
|
Node positive |
34.9 |
33.6 |
33.5 |
|
1-3 (#of nodes) |
24.4 |
24.4 |
24.3 |
|
4-9 |
7.5 |
6.4 |
6.8 |
|
>9 |
2.9 |
2.7 |
2.3 |
|
Tumor Grade (%) |
|||
|
Well-differentiated |
20.8 |
20.5 |
21.2 |
|
Moderately differentiated |
46.8 |
47.8 |
46.5 |
|
Poorly/undifferentiated |
23.7 |
23.3 |
23.7 |
|
Not assessed/recorded |
8.7 |
8.4 |
8.5 |
Figure 1 — Disease-Free Survival Kaplan Meier Survival Curve for all Patients Randomized to Anastrozole Tablets or Tamoxifen Monotherapy in the ATAC trial (Intent-to-Treat)

Figure 2 — Disease-free Survival for Hormone Receptor-Positive Subpopulation of Patients Randomized to Anastrozole Tablets or Tamoxifen Monotherapy in the ATAC Trial
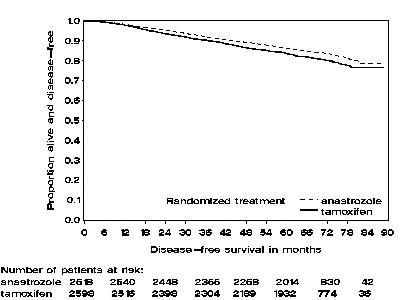
In the group of patients who had previous adjuvant chemotherapy (N=698 for anastrozole tablets and N=647 for tamoxifen), the hazard ratio for disease-free survival was 0.91(95% CI: 0.73 to 1.13) in the anastrozole tablets arm compared to the tamoxifen arm.
Table 8- All Recurrence and Death Events*
|
* The combination arm was discontinued due to lack of efficacy benefit at 33 months of follow-up † N=Number of patients randomized |
|||||
|
|
Intent-To-Treat Population‡
|
Hormone Receptor-Positive
Subpopulation‡ |
|||
|
|
Anastrozole tablets 1 mg
(N†=3125) |
Tamoxifen
20 mg (N†=3116) |
Anastrozole tablets 1 mg
(N†=2618) |
Tamoxifen
20 mg (N†=2598) |
|
| Median Duration of Therapy (mo) |
60
|
60
|
60
|
60
|
|
| Median Efficacy Follow-up (mo) |
68
|
68
|
68
|
68
|
|
| Loco-regional recurrence |
119 (3.8)
|
149 (4.8)
|
76 (2.9)
|
101 (3.9)
|
|
| Contralateral breast cancer |
35 (1.1)
|
59 (1.9)
|
26 (1.0)
|
54 (2.1)
|
|
| Invasive
|
27 (0.9)
|
52 (1.7)
|
21 (0.8)
|
48 (1.8)
|
|
| Ductal carcinoma in situ |
8 (0.3) |
6 (0.2) |
5 (0.2) |
5 (0.2) |
|
| Unknown |
0 |
1 (<0.1) |
0 |
1 (<0.1) |
|
| Distant recurrence |
324 (10.4)
|
375 (12.0)
|
226 (8.6)
|
265 (10.2)
|
|
| Death from Any Cause |
411 (13.2)
|
420 (13.5)
|
296 (11.3)
|
301 (11.6)
|
|
| Death breast cancer |
218 (7.0) |
248 (8.0) |
138 (5.3) |
160 (6.2) |
|
| Death other reason (including unknown) |
193 (6.2) |
172 (5.5) |
158 (6.0) |
141 (5.4) |
|
‡
Table 9 - ATAC Efficacy Summary*
|
* The combination arm was discontinued due to lack of efficacy benefit at 33 months of follow-up. |
||||
|
|
Intent-To-Treat Population
|
Hormone Receptor-Positive
Subpopulation |
||
|
|
Anastrozole tablets 1 mg
(N=3125) |
Tamoxifen
20 mg (N=3116) |
Anastrozole tablets 1 mg
(N=2618) |
Tamoxifen
20 mg (N=2598) |
|
|
Number of Events
|
Number of Events
|
||
|
Disease
free
Survival
|
575 |
651 |
424 |
497 |
| Hazard ratio |
0.87
|
0.83
|
||
| 2-sided 95% CI
|
0.78 to 0.97
|
0.73 to 0.94
|
||
| p-value
|
0.0127 |
0.0049 |
||
|
Distant
Disease-
free
Survival
|
500 |
530 |
370 |
394 |
| Hazard ratio |
0.94
|
0.93
|
||
| 2-sided 95% CI |
0.83 to 1.06
|
0.80 to 1.07
|
||
|
Overall
Survival
|
411 |
420 |
296 |
301 |
| Hazard ratio |
0.97
|
0.97
|
||
| 2-sided 95% CI |
0.85 to 1.12
|
0.83 to 1.14
|
||
14.2 First-Line Therapy in Postmenopausal Women with Advanced Breast Cancer
Table 10 – Demographic and Other Baseline Characteristics
|
* ER=Estrogen receptor † PgR=Progesterone receptor |
||||
|
|
Number (%) of subjects
|
|||
|
|
Trial 0030
|
Trial 0027
|
||
|
Receptor status
|
Anastrozole tablets 1 mg
(N=171) |
Tamoxifen
20 mg (N=182) |
Anastrozole tablets 1 mg
(N=340) |
Tamoxifen
20 mg (N=328) |
| ER* and/or PgR†
|
151 (88.3) |
162 (89.0) |
154 (45.3) |
144 (43.9) |
| ER* unknown, PgR†
Unknown |
19 (11.1) |
20 (11.0) |
185 (54.4) |
183 (55.8) |
Table -11 Efficacy Results of First –line Treatment
|
* LCL=Lower Confidence Limit † Tamoxifen:Anastrozole tablets ‡ CI=Confidence Interval § Two-sided Log Rank ¶ CR=Complete Response # PR=Partial Response ♠Anastrozole tablets:Tamoxifen |
||||
|
Endpoint |
Trial 0030 |
Trial 0027 |
||
|
|
Anastrozole tablets 1 mg (N=171) |
Tamoxifen 20 mg (N=182) |
Anastrozole tablets 1 mg (N=340) |
Tamoxifen 20 mg (N=328) |
|
Time to progression (TTP) Median TTP (months) |
11.1 |
5.6 |
8.2 |
8.3 |
|
Number (%) of subjects |
114 (67%) |
138 (76%) |
249 (73%) |
247 (75%) |
|
Who progressed Hazard ratio (LCL* )† |
1.42 (1.15) |
1.01 (0.87) |
||
|
2-sided 95% CI‡ |
(1.11, 1.82) |
(0.85, 1.20) |
||
|
p-value§ |
0.006 |
0.920 |
||
|
Best objective response rate Number (%) of subjects |
36 (21.1%) |
31 (17.0%) |
112 (32.9%) |
107 (32.6%) |
|
With CR¶ + PR# Odds Ratio (LCL* )♠ |
1.30 (0.83) |
1.01 (0.77) |
||
Figure 3 - Kaplan-Meier probability of time to disease progression for all randomized patients (intent-to-treat) in Trial 0030
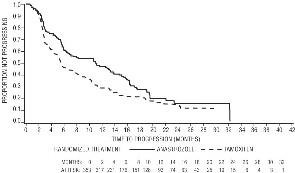
Figure 4 - Kaplan-Meier probability of time to progression for all randomized patients (intent-to-treat) in Trial 0027
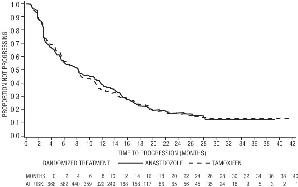
Results from the secondary endpoints were supportive of the results of the primary efficacy endpoints. There were too few deaths occurring across treatment groups of both trials to draw conclusions on overall survival differences.
14.3 Second-Line Therapy in Postmenopausal Women with Advanced Breast Cancer who had Disease Progression following Tamoxifen Therapy
Table 12– Efficacy Results of Second-line Treatment
|
*Surviving Patients |
|||
|
|
Anastrozole tablets 1 mg
|
Anastrozole tablets 10 mg
|
Megestrol Acetate
160 mg
|
| Trial 0004 (N. America) |
(N=128)
|
(N=130)
|
(N=128)
|
| Median Fol1ow-up (months)*
|
31.3
|
30.9
|
32.9
|
| Median Time to Death (months)
|
29.6
|
25.7
|
26.7
|
| 2 Year Survival Probability (%)
|
62.0
|
58.0
|
53.1
|
| Median Time to Progression (months)
|
5.7
|
5.3
|
5.1
|
| Objective Response (all patients ) (%) |
12.5
|
10.0
|
10.2
|
| Stable Disease for >24 weeks (%)
|
35.2
|
29.2
|
32.8
|
| Progression (%)
|
86.7
|
85.4
|
90.6
|
| Trial 0005 (Europe, Australia, S. Africa) |
(N=135)
|
(N=118)
|
(N=125)
|
| Median Follow-up (months)*
|
31.0 |
30.9 |
31.5 |
| Median Time to Death (months) |
24.3 |
24.8 |
19.8 |
| 2 Year Survival Probability (%) |
50.5 |
50.9 |
39.1 |
| Median Time to Progression (months) |
4.4 |
5.3 |
3.9 |
| Objective Response (all patients) (%) |
12.6 |
15.3 |
14.4 |
| Stable Disease for >24 weeks (%) |
24.4 |
25.4 |
23.2 |
| Progression (%) |
91.9 |
89.8 |
92.0 |
Table 13 – Pooled Efficacy Results of Second-line Treatment
|
Trials 0004 & 0005
(Pooled Data) |
Anastrozole tablets 1 mg
N=263 |
Anastrozole tablets 10 mg
N=248 |
Megestrol
Acetate 160 mg N=253 |
| Median Time to Death (months) |
26.7
|
25.5
|
22.5
|
| 2 Year Survival Probability (%) |
56.1
|
54.6
|
46.3
|
| Median Time to Progression |
4.8
|
5.3
|
4.6
|
| Objective Response (all patients) (%) |
12.5
|
12.5
|
12.3
|
16 HOW SUPPLIED/STORAGE AND HANDLING
These tablets are supplied in Box - Unit Dose of 30 tablets (NDC 0179-0068-70)
Storage
17 PATIENT COUNSELING INFORMATION
17.1 Pregnancy
17.2 Allergic (Hypersensitivity) Reactions
Patients should be informed of the possibility of serious allergic reactions with swelling of the face, lips, tongue and/or throat (angioedema) which may cause difficulty in swallowing and/or breathing and to immediately report this to their doctor.
17.3 Ischemic Cardiovascular Events
Patients with pre-existing ischemic heart disease should be informed that an increased incidence of cardiovascular events has been observed with anastrozole tablets use compared to tamoxifen use.
17.4 Bone Effects
17.5 Cholesterol
Patients should be informed that an increased level of cholesterol might be seen while receiving anastrozole tablets.
17.6 Tamoxifen
17.7 FDA-Approved Patient Labeling
PATIENT INFORMATION
What are anastrozole tablets?
- treatment of early breast cancer
- first treatment of locally advanced or metastatic breast cancer, in women whose breast cancer is hormone receptor-positive or the hormone receptors are not known.
- treatment of advanced breast cancer, if the cancer has grown, or the disease has spread after tamoxifen therapy.
Who should not take anastrozole tablets?
- are pregnant, think you may be pregnant, or plan to get pregnant. Anastrozole tablets may harm your unborn child. If you become pregnant while taking anastrozole tablets, tell your doctor right away.
- have not finished menopause (are premenopausal)
- are allergic to any of the ingredients in anastrozole tablets. See the end of this leaflet for a list of the ingredients in anastrozole tablets.
- are a man or child
What is the most important information I should know about anastrozole tablets?
-
Heart disease. Women with early breast cancer, who have a history of blockages in heart arteries (ischemic heart disease) and who take anastrozole tablets may have a slight increase in this type of heart disease compared to similar patients who take tamoxifen.
- Stop taking anastrozole tablets and call your doctor right away if you have chest pain or shortness of breath. These can be symptoms of heart disease.
- Osteoporosis (bone softening and weakening). Anastrozole tablets lower estrogen in your body, which may cause your bones to become softer and weaker. This can increase your chance of fractures, specifically of the spine, hip and wrist. Your doctor may order a test for you called a bone mineral density study before you start taking anastrozole tablets and during treatment with anastrozole tablets as needed.
What should I tell my doctor before taking anastrozole tablets?
Anastrozole tablets may not be right for you. Before taking anastrozole tablets, tell your doctor about all your medical conditions, including if you:
- have not finished menopause. Talk to your doctor if you are not sure. See “Who should not take anastrozole tablets?”
- have had a previous heart problem
- have a condition called osteoporosis
- have high cholesterol
- are pregnant, planning to become pregnant, or breast feeding. See “Who should not take anastrozole tablets?”
- are nursing a baby. It is not known if anastrozole tablets pass into breast milk. You and your doctor should decide if you will take anastrozole tablets or breast feed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Especially tell your doctor if you take:
- Tamoxifen. You should not take anastrozole tablets with tamoxifen. Taking tamoxifen with anastrozole tablets may lower the amount of anastrozole tablets in your blood and may cause anastrozole tablets not to work as well.
-
Medicines containing estrogen. Anastrozole tablets may not work if taken with one of these medicines:
- hormone replacement therapy
- birth control pills
- estrogen creams
- vaginal rings
- vaginal suppositories
How should I take anastrozole tablets?
- Take anastrozole tablets exactly as prescribed by your doctor. Keep taking anastrozole tablets for as long as your doctor prescribes it for you.
- Take one anastrozole tablet each day.
- Anastrozole tablets can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Take your next regularly scheduled dose. Do not take two doses at the same time.
- If you have taken more anastrozole tablets than your doctor has prescribed, contact your doctor right away. Do not take any additional anastrozole tablets until instructed to do so by your doctor.
Talk with your doctor about any health changes you have while taking anastrozole tablets.
What are possible side effects of anastrozole tablets?
Anastrozole tablets can cause serious side effects including:
- See “What is the most important information I should know about anastrozole tablets?”
- increased blood cholesterol (fat in the blood). Your doctor may check your cholesterol while you take anastrozole tablets therapy.
- skin reactions. Stop taking anastrozole tablets and call your doctor right away if you get any skin lesions, ulcers, or blisters.
- severe allergic reactions. Get medical help right away if you have:
· swelling of the face, lips, tongue, or throat.
· trouble swallowing
· trouble breathing
- liver problems. Anastrozole tablets can cause inflammation of the liver and changes in blood tests of the liver function. Your doctor may monitor you for this. Stop taking anastrozole tablets and call your doctor right away if you have any of these signs or symptoms of a liver problem:
· a general feeling of not being well
· yellowing of the skin or whites of the eyes
· pain on the right side of your abdomen
· hot flashes
· weakness
· joint pain
· carpal tunnel syndrome (tingling, pain, coldness, weakness in parts of the hand)
· pain
· sore throat
· mood changes
· high blood pressure
· depression
· nausea and vomiting
· thinning of the hair (hair loss)
· rash
· back pain
· sleep problems
· bone pain
· headache
· swelling
· increased cough
· shortness of breath
· lymphedema (build up of lymph fluid in the tissues of your affected arm)
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
HOW SHOULD I STORE ANASTROZOLE TABLETS?
- Store anastrozole tablets at 68ºF to 77ºF (20ºC to 25ºC).
- Keep anastrozole tablets and all medicines out of the reach of children.
General information about anastrozole tablets.
What are the ingredients in anastrozole tablets?
Anastrozole tablets 1mg - 30 tablets in a Box-Unit Dose
Anastrozole Tablets 1 mg - Box-Unit Dose of 30 tablets
RX Only
NDC 0179-0068-70
Each tablet contains 1 mg of anastrozole USP
USUAL DOSAGE: See accompanying Prescribing Information.
WARNING: As with all medications, keep out of the reach of children.
ANASTROZOLEANASTROZOLE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||