Atenolol and Chlorthalidone
Atenolol and ChlorthalidoneTablets USPRevised: December 2012Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- ATENOLOL AND CHLORTHALIDONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ATENOLOL AND CHLORTHALIDONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ATENOLOL AND CHLORTHALIDONE ADVERSE REACTIONS
- SPL UNCLASSIFIED
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ATENOLOL AND CHLORTHALIDONE DESCRIPTION
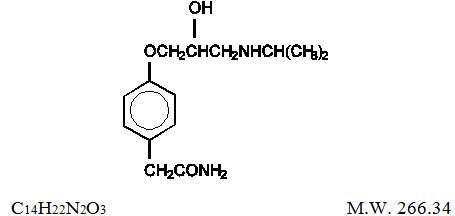
Atenolol and chlorthalidone tablets are for the treatment of hypertension. It combines the antihypertensive activity of two agents: a beta1-selective (cardioselective) hydrophilic blocking agent (atenolol), and a monosulfonamyl diuretic (chlorthalidone). Atenolol is Benzeneacetamide, 4-[2-hydroxy-3-[(1-methylethyl)amino]propoxy]-. It has the following structural formula:
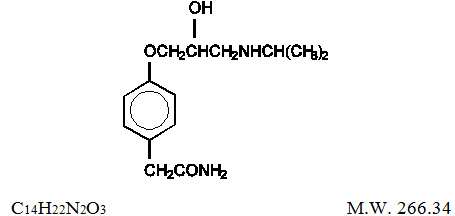
Atenolol (free base) is a relatively polar hydrophilic compound with a water solubility of 26.5 mg/mL at 37°C. It is freely soluble in 1N HCl (300 mg/mL at 25°C) and less soluble in chloroform (3 mg/mL at 25°C).
Chlorthalidone is 2-Chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl)benzenesulfonamide. Chlorthalidone has a water solubility of 12 mg/100 mL at 20°C. It has the following structural formula:
Each atenolol and chlorthalidone tablet 50 mg-25 mg for oral administration contains: atenolol USP, 50 mg and chlorthalidone USP, 25 mg.
Each atenolol and chlorthalidone tablet 100 mg-25 mg for oral administration contains: atenolol USP, 100 mg and chlorthalidone USP, 25 mg.
Atenolol and Chlorthalidone Tablets USP, 50 mg-25 mg and 100 mg-25 mg, contain the following inactive ingredients: magnesium stearate, microcrystalline cellulose, povidone and sodium starch glycolate.
CLINICAL PHARMACOLOGY
Atenolol and chlorthalidone have been used singly and concomitantly for the treatment of hypertension. The antihypertensive effects of these agents are additive, and studies have shown that there is no interference with bioavailability when these agents are given together in the single combination tablet. Therefore, this combination provides a convenient formulation for the concomitant administration of these two entities. In patients with more severe hypertension, atenolol and chlorthalidone may be administered with other antihypertensives such as vasodilators.
Atenolol is a beta1-selective (cardioselective) beta-adrenergic receptor blocking agent without membrane stabilizing or intrinsic sympathomimetic (partial agonist) activities. This preferential effect is not absolute, however, and at higher doses, atenolol inhibits beta2-adrenoreceptors, chiefly located in the bronchial and vascular musculature.
In standard animal or human pharmacological tests, beta-adrenoreceptor blocking activity of atenolol has been demonstrated by: (1) reduction in resting and exercise heart rates and cardiac output, (2) reduction of systolic and diastolic blood pressure at rest and on exercise, (3) inhibition of isoproterenol induced tachycardia and (4) reduction in reflex orthostatic tachycardia.
A significant beta-blocking effect of atenolol, as measured by reduction of exercise tachycardia, is apparent within one hour following oral administration of a single dose. This effect is maximal at about 2 to 4 hours and persists for at least 24 hours. The effect at 24 hours is dose related and also bears a linear relationship to the logarithm of plasma atenolol concentration. However, as has been shown for all beta-blocking agents, the antihypertensive effect does not appear to be related to plasma level.
In normal subjects, the beta1-selectivity of atenolol has been shown by its reduced ability to reverse the beta2-mediated vasodilating effect of isoproterenol as compared to equivalent beta-blocking doses of propranolol. In asthmatic patients, a dose of atenolol producing a greater effect on resting heart rate than propranolol resulted in much less increase in airway resistance. In a placebo-controlled comparison of approximately equipotent oral doses of several beta blockers, atenolol produced a significantly smaller decrease of FEV1 than nonselective beta blockers, such as propranolol and unlike those agents did not inhibit bronchodilation in response to isoproterenol.
Consistent with its negative chronotropic effect due to beta blockade of the SA node, atenolol
increases sinus cycle length and sinus node recovery time. Conduction in the AV node is also prolonged. Atenolol is devoid of membrane stabilizing activity, and increasing the dose well beyond that producing beta blockade does not further depress myocardial contractility. Several studies have demonstrated a moderate (approximately 10%) increase in stroke volume at rest and exercise.
In controlled clinical trials, atenolol given as a single daily dose, was an effective antihypertensive agent providing 24-hour reduction of blood pressure. Atenolol has been studied in combination with thiazide-type diuretics and the blood pressure effects of the combination are approximately additive. Atenolol is also compatible with methyldopa, hydralazine and prazosin, the combination resulting in a larger fall in blood pressure than with the single agents. The dose range of atenolol is narrow, and increasing the dose beyond 100 mg once daily is not associated with increased antihypertensive effect. The mechanisms of the antihypertensive effects of beta-blocking agents have not been established. Several mechanisms have been proposed and include: (1) competitive antagonism of catecholamines at peripheral (especially cardiac) adrenergic neuron sites, leading to decreased cardiac output, (2) a central effect leading to reduced sympathetic outflow to the periphery and (3) suppression of renin activity. The results from long-term studies have not shown any diminution of the antihypertensive efficacy of atenolol with prolonged use.
In man, absorption of an oral dose is rapid and consistent but incomplete. Approximately 50% of an oral dose is absorbed from the gastrointestinal tract, the remainder being excreted unchanged in the feces. Peak blood levels are reached between 2 and 4 hours after ingestion. Unlike propranolol or metoprolol, but like nadolol, hydrophilic atenolol undergoes little or no metabolism by the liver, and the absorbed portion is eliminated primarily by renal excretion. Atenolol also differs from propranolol in that only a small amount (6-16%) is bound to proteins in the plasma. This kinetic profile results in relatively consistent plasma drug levels with about a fourfold interpatient variation. There is no information as to the pharmacokinetic effect of atenolol on chlorthalidone.
The elimination half-life of atenolol is approximately 6 to 7 hours and there is no alteration of the kinetic profile of the drug by chronic administration. Following doses of 50 mg or 100 mg, both beta-blocking and antihypertensive effects persist for at least 24 hours. When renal function is impaired, elimination of atenolol is closely related to the glomerular filtration rate; but significant accumulation does not occur until the creatinine clearance falls below 35 mL/min/1.73 m2 (see prescribing information for atenolol).
In general, elderly patients present higher atenolol plasma levels with total clearance values about 50% lower than younger subjects. The half-life is markedly longer in the elderly compared to younger subjects. The reduction of atenolol clearance follows the general trend that elimination of renally excreted drugs is decreased with increasing age.
Chlorthalidone is a monosulfonamyl diuretic which differs chemically from thiazide diuretics in that a double ring system is incorporated in its structure. It is an oral diuretic with prolonged action and low toxicity. The diuretic effect of the drug occurs within 2 hours of an oral dose. It produces diuresis with greatly increased excretion of sodium and chloride. At maximal therapeutic dosage, chlorthalidone is approximately equal in its diuretic effect to comparable maximal therapeutic doses of benzothiadiazine diuretics. The site of action appears to be the cortical diluting segment of the ascending limb of Henle’s loop of the nephron.
INDICATIONS & USAGE
Atenolol and chlorthalidone is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including atenolol and chlorthalidone.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (eg, n angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
This fixed dose combination drug is not indicated for initial therapy of hypertension. If the fixed dose combination represents the dose appropriate to the individual patient's needs, it may be more convenient than the separate components.
ATENOLOL AND CHLORTHALIDONE CONTRAINDICATIONS
Atenolol and chlorthalidone tablets are contraindicated in patients with: sinus bradycardia; heart block greater than first degree; cardiogenic shock; overt cardiac failure (see WARNINGS ); anuria; hypersensitivity to this product or to sulfonamide-derived drugs.
WARNINGS
Sympathetic stimulation is necessary in supporting circulatory function in congestive heart failure, and beta blockade carries the potential hazard of further depressing myocardial contractility and precipitating more severe failure.
IN PATIENTS WITHOUT A HISTORY OF CARDIAC FAILURE, continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first sign or symptom of impending cardiac failure, patients should be treated appropriately according to currently recommended guidelines, and the response observed closely. If cardiac failure continues despite adequate treatment, atenolol and chlorthalidone should be withdrawn. (See DOSAGE AND ADMINISTRATION .)
Since atenolol is excreted via the kidneys, atenolol and chlorthalidone should be used with caution in patients with impaired renal function.
In patients with renal disease, thiazides may precipitate azotemia. Since cumulative effects may develop in the presence of impaired renal function, if progressive renal impairment becomes evident, atenolol and chlorthalidone should be discontinued.
In patients with impaired hepatic function or progressive liver disease, minor alterations in fluid and electrolyte balance may precipitate hepatic coma. Atenolol and chlorthalidone should be used with caution in these patients.
Following abrupt cessation of therapy with certain beta-blocking agents in patients with coronary artery disease, exacerbations of angina pectoris and, in some cases, myocardial infarction have been reported. Therefore, such patients should be cautioned against interruption of therapy without the physician’s advice. Even in the absence of overt angina pectoris, when discontinuation of atenolol and chlorthalidone is planned, the patient should be carefully observed and should be advised to limit physical activity to a minimum. Atenolol and chlorthalidone should be reinstated if withdrawal symptoms occur. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue atenolol and chlorthalidone therapy abruptly even in patients treated only for hypertension.
Bradycardia and heart block can occur and the left ventricular end diastolic pressure can rise when beta-blockers are administered with verapamil or diltiazem. Patients with pre-existing conduction abnormalities or left ventricular dysfunction are particularly susceptible. (See PRECAUTIONS .)
PATIENTS WITH BRONCHOSPASTIC DISEASE SHOULD, IN GENERAL, NOT RECEIVE BETA BLOCKERS. Because of its relative beta 1 -selectivity, however, atenolol and chlorthalidone may be used with caution in patients with bronchospastic disease who do not respond to or cannot tolerate, other antihypertensive treatment. Since beta 1 -selectivity is not absolute, the lowest possible dose of atenolol and chlorthalidone should be used and a beta 2 -stimulating agent (bronchodilator) should be made available. If dosage must be increased, dividing the dose should be considered in order to achieve lower peak blood levels.
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Atenolol and chlorthalidone may be used with caution in diabetic patients. Beta blockers may mask tachycardia occurring with hypoglycemia, but other manifestations such as dizziness and sweating may not be significantly affected. At recommended doses atenolol does not potentiate insulin-induced hypoglycemia and, unlike nonselective beta blockers, does not delay recovery of blood glucose to normal levels.
Insulin requirements in diabetic patients may be increased, decreased or unchanged; latent diabetes mellitus may become manifest during chlorthalidone administration.
Beta-adrenergic blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Abrupt withdrawal of beta blockade might precipitate a thyroid storm; therefore, patients suspected of developing thyrotoxicosis from whom atenolol and chlorthalidone therapy is to be withdrawn should be monitored closely.
Because calcium excretion is decreased by thiazides, atenolol and chlorthalidone should be discontinued before carrying out tests for parathyroid function. Pathologic changes in the
parathyroid glands, with hypercalcemia and hypophosphatemia, have been observed in a few patients on prolonged thiazide therapy; however, the common complications of hyperparathyroidism such as renal lithiasis, bone resorption, and peptic ulceration have not been seen.
Hyperuricemia may occur, or acute gout may be precipitated in certain patients receiving thiazide therapy.
Atenolol and chlorthalidone tablets should not be given to patients with untreated pheochromocytoma.
Atenolol can cause fetal harm when administered to a pregnant woman. Atenolol crosses the placental barrier and appears in cord blood. Administration of atenolol, starting in the second trimester of pregnancy, has been associated with the birth of infants that are small for gestational age. No studies have been performed on the use of atenolol in the first trimester and the possibility of fetal injury cannot be excluded. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Neonates born to mothers who are receiving atenolol at parturition or breastfeeding may be at risk for hypoglycemia and bradycardia. Caution should be exercised when atenolol and chlorthalidone is administered during pregnancy or to a woman who is breastfeeding. (See PRECAUTIONS, Nursing Mothers .)
Atenolol and chlorthalidone was studied for teratogenic potential in the rat and rabbit. Doses of atenolol/chlorthalidone of 8/2, 80/20 and 240/60 mg/kg/day were administered orally to pregnant rats with no evidence of embryofetotoxicity observed. Two studies were conducted in rabbits. In the first study, pregnant rabbits were dosed with 8/2, 80/20 and 160/40 mg/kg/day of atenolol/chlorthalidone. No teratogenic effects were noted, but embryonic resorptions were observed at all dose levels (ranging from approximately 5 times to 100 times the maximum recommended human dose*). In the second rabbit study, doses of atenolol/chlorthalidone were 4/1, 8/2 and 20/5 mg/kg/day. No teratogenic or embryotoxic effects were demonstrated.
Atenolol
Atenolol has been shown to produce a dose-related increase in embryo/fetal resorptions in rats at doses equal to or greater than 50 mg/kg/day or 25 or more times the maximum recommended human antihypertensive dose.* Although similar effects were not seen in rabbits, the compound was not evaluated in rabbits at doses above 25 mg/kg/day or 12.5 times the maximum recommended human antihypertensive dose.*
*Based on the maximum dose of 100 mg/day in a 50 kg patient.
Chlorthalidone
Thiazides cross the placental barrier and appear in cord blood. The use of chlorthalidone and related drugs in pregnant women requires that the anticipated benefits of the drug be weighed against possible hazards to the fetus. These hazards include fetal or neonatal jaundice, thrombocytopenia and possibly other adverse reactions which have occurred in the adult.
PRECAUTIONS
Atenolol and chlorthalidone tablets may aggravate peripheral arterial circulatory disorders.
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
Patients should be observed for clinical signs of fluid or electrolyte imbalance; i.e., hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are
particularly important when the patient is vomiting excessively or receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance include dryness of the mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Measurement of potassium levels is appropriate especially in elderly patients, those receiving digitalis preparations for cardiac failure, patients whose dietary intake of potassium is abnormally low, or those suffering from gastrointestinal complaints.
Hypokalemia may develop especially with brisk diuresis, when severe cirrhosis is present, or during concomitant use of corticosteroids or ACTH.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia.
Hypokalemia can sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased ventricular irritability). Hypokalemia may be avoided or treated by use of potassium supplements or foods with a high potassium content.
Any chloride deficit during thiazide therapy is generally mild and usually does not require specific treatment except under extraordinary circumstances (as in liver disease or renal disease). Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction rather than administration of salt except in rare instances when the hyponatremia is life-threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Atenolol and chlorthalidone may potentiate the action of other antihypertensive agents used concomitantly. Patients treated with atenolol and chlorthalidone plus a catecholamine depletor (e.g., reserpine) should be closely observed for evidence of hypotension and/or marked bradycardia which may produce vertigo, syncope, or postural hypotension.
Calcium channel blockers may also have an additive effect when given with atenolol and chlorthalidone. (See WARNINGS .)
Disopyramide is a Type I antiarrhythmic drug with potent negative inotropic and chronotropic effects. Disopyramide has been associated with severe bradycardia, asystole and heart failure when administered with beta blockers.
Amiodarone is an antiarrhythmic agent with negative chronotropic properties that may be additive to those seen with beta blockers.
Thiazides may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude the therapeutic effectiveness of norepinephrine. Thiazides may increase the responsiveness to tubocurarine.
Concomitant use of prostaglandin synthase inhibiting drugs, e.g., indomethacin, may decrease the hypotensive effects of beta blockers.
Lithium generally should not be given with diuretics because they reduce its renal clearance and add a high risk of lithium toxicity. Read prescribing information for lithium preparations before use of such preparations with atenolol and chlorthalidone.
Beta blockers may exacerbate the rebound hypertension which can follow the withdrawal of clonidine. If the two drugs are coadministered, the beta blocker should be withdrawn several days before the gradual withdrawal of clonidine. If replacing clonidine by beta-blocker therapy, the introduction of beta blockers should be delayed for several days after clonidine administration has stopped.
While taking beta blockers, patients with a history of anaphylactic reaction to a variety of allergens may have a more severe reaction on repeated challenge, either accidental, diagnostic or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat the allergic reaction.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
In patients receiving thiazides, sensitivity reactions may occur with or without a history of allergy or bronchial asthma. The possible exacerbation or activation of systemic lupus erythematosus has been reported. The antihypertensive effects of thiazides may be enhanced in the postsympathectomy patient.
Two long-term (maximum dosing duration of 18 or 24 months) rat studies and one long-term (maximum dosing duration of 18 months) mouse study, each employing dose levels as high as 300 mg/kg/day or 150 times the maximum recommended human antihypertensive dose*, did not indicate a carcinogenic potential of atenolol. A third (24 month) rat study, employing doses of 500 and 1,500 mg/kg/day (250 and 750 times the maximum recommended human antihypertensive dose*) resulted in increased incidences of benign adrenal medullary tumors in males and females, mammary fibroadenomas in females, and anterior pituitary adenomas and thyroid parafollicular cell carcinomas in males. No evidence of a mutagenic potential of atenolol was uncovered in the dominant lethal test (mouse), in vivo cytogenetics test (Chinese hamster) or Ames test (S typhimurium).
*Based on the maximum dose of 100 mg/day in a 50 kg patient.
Fertility of male or female rats (evaluated at dose levels as high as 200 mg/kg/day or 100 times the maximum recommended human dose*) was unaffected by atenolol administration.
Six month oral administration studies were conducted in rats and dogs using atenolol and chlorthalidone doses up to 12.5 mg/kg/day (atenolol/chlorthalidone 10/2.5 mg/kg/day–approximately five times the maximum recommended human antihypertensive dose*). There were no functional or morphological abnormalities resulting from dosing either compound alone or together other than minor changes in heart rate, blood pressure and urine chemistry which were attributed to the known pharmacologic properties of atenolol and/or chlorthalidone.
Chronic studies of atenolol performed in animals have revealed the occurrence of vacuolation of epithelial cells of Brunner’s glands in the duodenum of both male and female dogs at all tested dose levels (starting at 15 mg/kg/day or 7.5 times the maximum recommended human antihypertensive dose*) and increased incidence of atrial degeneration of hearts of male rats at 300 but not 150 mg atenolol/kg/day (150 and 75 times the maximum recommended human antihypertensive dose,* respectively).
*Based on the maximum dose of 100 mg/day in a 50 kg patient.
Pregnancy Category D: See WARNINGS-Pregnancy and Fetal Injury .
Atenolol is excreted in human breast milk at a ratio of 1.5 to 6.8 when compared to the concentration in plasma. Caution should be exercised when atenolol is administered to a nursing woman. Clinically significant bradycardia has been reported in breastfed infants. Premature infants, or infants with impaired renal function, may be more likely to develop adverse effects.
Neonates born to mothers who are receiving atenolol at parturition or breastfeeding may be at risk for hypoglycemia and bradycardia. Caution should be exercised when atenolol and chlorthalidone is administered during pregnancy or to a woman who is breastfeeding. (See WARNINGS, Pregnancy and Fetal Injury .)
Safety and effectiveness in pediatric patients have not been established.
Clinical studies of atenolol and chlorthalidone did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy.
ATENOLOL AND CHLORTHALIDONE ADVERSE REACTIONS
Atenolol and chlorthalidone tablets are usually well tolerated in properly selected patients. Most adverse effects have been mild and transient. The adverse effects observed for atenolol and chlorthalidone are essentially the same as those seen with the individual components.
The frequency estimates in the following table were derived from controlled studies in which adverse reactions were either volunteered by the patient (US studies) or elicited, e.g., by checklist (foreign studies). The reported frequency of elicited adverse effects was higher for both atenolol and placebo-treated patients than when these reactions were volunteered. Where frequency of adverse effects for atenolol and placebo is similar, causal relationship to atenolol is uncertain.
| Volunteered | Total–Volunteered and Elicited | |||
| (US Studies) | (Foreign + US Studies) | |||
| Atenolol | Placebo | Atenolol | Placebo | |
| (n=164) | (n=206) | (n=399) | (n=407) | |
| % | % | % | % | |
| CARDIOVASCULAR | ||||
| Bradycardia | 3 | 0 | 3 | 0 |
| Cold Extremities | 0 | 0.5 | 12 | 5 |
| Postural Hypotension | 2 | 1 | 4 | 5 |
| Leg Pain | 0 | 0.5 | 3 | 1 |
| CENTRAL NERVOUS SYSTEM/ | ||||
| NEUROMUSCULAR | ||||
| Dizziness | 4 | 1 | 13 | 6 |
| Vertigo | 2 | 0.5 | 2 | 0.2 |
| Light-Headedness | 1 | 0 | 3 | 0.7 |
| Tiredness | 0.6 | 0.5 | 26 | 13 |
| Fatigue | 3 | 1 | 6 | 5 |
| Lethargy | 1 | 0 | 3 | 0.7 |
| Drowsiness | 0.6 | 0 | 2 | 0.5 |
| Depression | 0.6 | 0.5 | 12 | 9 |
| Dreaming | 0 | 0 | 3 | 1 |
| GASTROINTESTINAL | ||||
| Diarrhea | 2 | 0 | 3 | 2 |
| Nausea | 4 | 1 | 3 | 1 |
| RESPIRATORY (see WARNINGS ) | ||||
| Wheeziness | 0 | 0 | 3 | 3 |
| Dyspnea | 0.6 | 1 | 6 | 4 |
During postmarketing experience, the following have been reported in temporal relationship to the use of the drug: elevated liver enzymes and/or bilirubin, hallucinations, headache, impotence, Peyronie’s disease, postural hypotension which may be associated with syncope, psoriasiform rash or exacerbation of psoriasis, psychoses, purpura, reversible alopecia, thrombocytopenia, visual disturbance, sick sinus syndrome and dry mouth. Atenolol, like other beta blockers, has been associated with the development of antinuclear antibodies (ANA), lupus syndrome and Raynaud’s phenomenon.
Cardiovascular: orthostatic hypotension; Gastrointestinal: anorexia, gastric irritation, vomiting, cramping, constipation, jaundice (intrahepatic cholestatic jaundice), pancreatitis; CNS: vertigo, paresthesia, xanthopsia; Hematologic: leukopenia, agranulocytosis, thrombocytopenia, aplastic anemia; Hypersensitivity: purpura, photosensitivity, rash, urticaria, necrotizing angiitis (vasculitis) [cutaneous vasculitis], Lyell’s syndrome (toxic epidermal necrolysis); Miscellaneous: hyperglycemia, glycosuria, hyperuricemia, muscle spasm, weakness, restlessness. Clinical trials of atenolol and chlorthalidone conducted in the United States (89 patients treated with atenolol and chlorthalidone) revealed no new or unexpected adverse effects.
SPL UNCLASSIFIED
In addition, a variety of adverse effects not observed in clinical trials with atenolol but reported with other beta-adrenergic blocking agents should be considered potential adverse effects of atenolol. Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, decreased performance on neuropsychometrics; Cardiovascular: Intensification of AV block (see CONTRAINDICATIONS ); Gastrointestinal: Mesenteric arterial thrombosis, ischemic colitis; Hematologic: Agranulocytosis; Allergic: Erythematous rash, fever combined with aching and sore throat, laryngospasm and respiratory distress.
There have been reports of skin rashes and/or dry eyes associated with the use of beta-adrenergic blocking drugs. The reported incidence is small, and, in most cases, the symptoms have cleared when treatment was withdrawn. Discontinuance of the drug should be considered if any such reaction is not otherwise explicable. Patients should be closely monitored following cessation of therapy. (See DOSAGE AND ADMINISTRATION .)
The oculomucocutaneous syndrome associated with the beta blocker practolol has not been reported with atenolol. Furthermore, a number of patients who had previously demonstrated established practolol reactions were transferred to atenolol therapy with subsequent resolution or quiescence of the reaction.
Clinically important changes in standard laboratory parameters were rarely associated with the administration of atenolol and chlorthalidone. The changes in laboratory parameters were not progressive and usually were not associated with clinical manifestations. The most common changes were increases in uric acid and decreases in serum potassium.
OVERDOSAGE
No specific information is available with regard to overdosage and atenolol and chlorthalidone in humans. Treatment should be symptomatic and supportive and directed to the removal of any unabsorbed drug by induced emesis, or administration of activated charcoal. Atenolol can be removed from the general circulation by hemodialysis. Further consideration should be given to dehydration, electrolyte imbalance and hypotension by established procedures.
Overdosage with atenolol has been reported with patients surviving acute doses as high as 5 g. One death was reported in a man who may have taken as much as 10 g acutely.
The predominant symptoms reported following atenolol overdose are lethargy, disorder of respiratory drive, wheezing, sinus pause, and bradycardia. Additionally, common effects associated with overdosage of any beta-adrenergic blocking agent are congestive heart failure, hypotension, bronchospasm, and/or hypoglycemia. Other treatment modalities should be employed at the physician’s discretion and may include:
BRADYCARDIA: Atropine 1-2 mg intravenously. If there is no response to vagal blockade, give isoproterenol cautiously. In refractory cases, a transvenous cardiac pacemaker may be indicated. Glucagon in a 10 mg intravenous bolus has been reported to be useful. If required, this may be repeated or followed by an intravenous infusion of glucagon 1-10 mg/h depending on response.
HEART BLOCK (SECOND OR THIRD DEGREE): Isoproterenol or transvenous pacemaker.
CONGESTIVE HEART FAILURE: Digitalize the patient and administer a diuretic. Glucagon has been reported to be useful.
HYPOTENSION: Vasopressors such as dopamine or norepinephrine (levarterenol). Monitor blood pressure continuously.
BRONCHOSPASM: A beta2-stimulant such as isoproterenol or terbutaline and/or aminophylline.
HYPOGLYCEMIA: Intravenous glucose.
ELECTROLYTE DISTURBANCE: Monitor electrolyte levels and renal function. Institute measures to maintain hydration and electrolytes.
Based on the severity of symptoms, management may require intensive support care and facilities for applying cardiac and respiratory support.
Symptoms of chlorthalidone overdose include nausea, weakness, dizziness and disturbances of electrolyte balance.
DOSAGE & ADMINISTRATION
DOSAGE MUST BE INDIVIDUALIZED (SEE INDICATIONS AND USAGE ): Chlorthalidone is usually given at a dose of 25 mg daily; the usual initial dose of atenolol is 50 mg daily. Therefore, the initial dose should be one atenolol and chlorthalidone 50 mg-25 mg tablet given once a day. If an optimal response is not achieved, the dosage should be increased to one atenolol and chlorthalidone 100 mg-25 mg tablet given once a day.
When necessary, another antihypertensive agent may be added gradually beginning with 50% of the usual recommended starting dose to avoid an excessive fall in blood pressure.
Since atenolol is excreted via the kidneys, dosage should be adjusted in cases of severe impairment of renal function. No significant accumulation of atenolol occurs until creatinine clearance falls below 35 mL/min/1.73 m2 (normal range is 100-150 mL/min/1.73 m2); therefore, the following maximum dosages are recommended for patients with renal impairment.
| Creatinine Clearance | Atenolol Elimination | |
| (mL/min/1.73 m2) | Half-life (hrs) | Maximum Dosage |
| 15-35 | 16-27 | 50 mg daily |
| <15 | >27 | 50 mg every other day |
HOW SUPPLIED
Atenolol and Chlorthalidone Tablets USP, 50 mg-25 mg are 10/32”, scored, round, white tablets imprinted DAN 5782 supplied in bottles of 100.
Atenolol and Chlorthalidone Tablets USP, 100 mg-25 mg are 12/32”, unscored, round, white tablets imprinted DAN 5783 supplied in bottles of 100.
Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature.] Dispense in a well-closed, light-resistant container with a child-resistant closure. Protect from heat, light and moisture. Protect from heat, light and moisture.
Manufactured by:
Watson Laboratories, Inc.
Corona, CA 92880 USA
Distributed by:
Watson Pharma, Inc.
Parsippany, NJ 07054 USA
Revised: December 2012
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
DRUG: Atenolol and Chlorthalidone
GENERIC: Atenolol and Chlorthalidone
DOSAGE: TABLET
ADMINSTRATION: ORAL
NDC: 52125-514-20
ACTIVE INGREDIENT(S):
- ATENOLOL 50mg in 1
- CHLORTHALIDONE 25mg in 1
INACTIVE INGREDIENT(S):
- CELLULOSE, MICROCRYSTALLINE
- POVIDONE K29/32
- MAGNESIUM STEARATE
- SODIUM STARCH GLYCOLATE TYPE A POTATO
COLOR: white
SHAPE: ROUND
SCORE: Two even pieces
SIZE: 8 mm
IMPRINT: DAN;5782
PACKAGING: 100 in 1 VIAL


Atenolol and ChlorthalidoneAtenolol and Chlorthalidone TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||