Atropine Sulfate
General Injectables & Vaccines, Inc
Atropine Sulfate Injection, USP FOR IM, IV, OR SC USE Rx Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- ATROPINE SULFATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- ATROPINE SULFATE INDICATIONS AND USAGE
- ATROPINE SULFATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ATROPINE SULFATE ADVERSE REACTIONS
- OVERDOSAGE
- ATROPINE SULFATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Sample Package Label
FULL PRESCRIBING INFORMATION
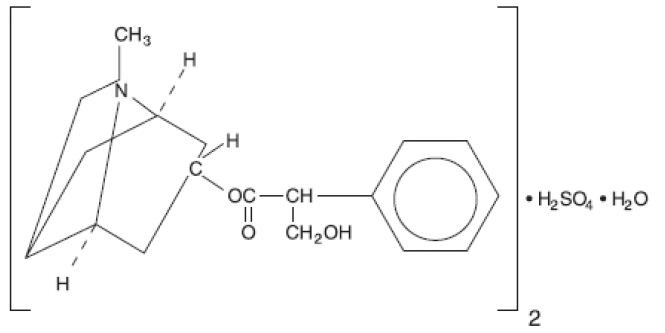
ATROPINE SULFATE DESCRIPTION
CLINICAL PHARMACOLOGY
ATROPINE SULFATE INDICATIONS AND USAGE
ATROPINE SULFATE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
General
Information for Patients
Drug Interactions
Pregnancy Category C
Nursing Mothers
ATROPINE SULFATE ADVERSE REACTIONS
OVERDOSAGE
Symptoms
Treatment
ATROPINE SULFATE DOSAGE AND ADMINISTRATION
| 7 - 16 lbs. - 0.1 mg |
40 - 65 lbs. - 0.3 mg |
| 17 - 24 lbs. - 0.15 mg |
65 - 90 lbs. - 0.4 mg |
| 24 - 40 lbs. - 0.2 mg |
Over 90 lbs. - 0.4 to 0.6 mg |
| mg required |
1 mg per mL |
0.4 mg per mL |
| 1 |
1 |
|
| 0.80 |
0.80 |
|
| 0.60 |
0.60 |
|
| 0.50 |
0.50 |
|
| 0.40 |
0.40 |
1 |
| 0.30 |
0.30 |
0.75 |
| 0.25 |
0.25 |
0.63 |
| 0.20 |
0.20 |
0.50 |
| 0.18 |
0.18 |
0.45 |
| 0.15 |
0.15 |
0.38 |
| 0.12 |
0.12 |
0.30 |
| 0.10 |
0.10 |
0.25 |
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
0.4 mg/mL
Store at 20°-25°C (68°-77°F), excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature].
www.fda.gov/medwatch
Sample Package Label

Atropine SulfateAtropine Sulfate INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!