Azithromycin
Azithromycin Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- AZITHROMYCIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- AZITHROMYCIN INDICATIONS AND USAGE
- AZITHROMYCIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AZITHROMYCIN ADVERSE REACTIONS
- AZITHROMYCIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- Adult Patients
- ANIMAL TOXICOLOGY
- PRINCIPAL DISPALY PANEL AZITHROMYCIN TABLETS 250MG
FULL PRESCRIBING INFORMATION
AZITHROMYCIN DESCRIPTION
D-xylo3872212
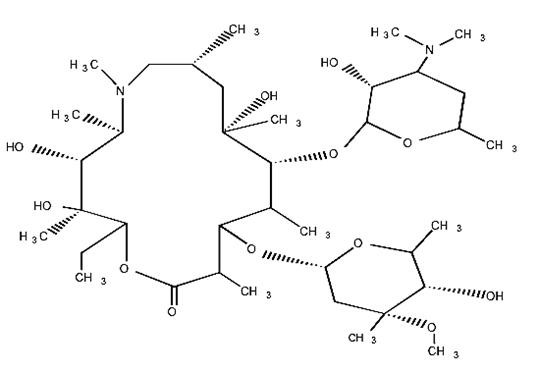 3872212
3872212CLINICAL PHARMACOLOGY
Pharmacokinetics
0-72maxmaxminmax
Day 1Day 5
max
max
0-24
min
0–∞
max
0–∞
1/2
0-288
max
max
max
AZITHROMYCIN CONCENTRATIONS FOLLOWING A 500 mg DOSE (TWO 250 mg CAPSULES) IN ADULTS1
| TISSUE OR FLUID | TIME AFTER DOSE (h) |
TISSUE OR FLUID CONCENTRATION (mcg/g or mcg/mL) |
CORRESPONDING PLASMA OR SERUM LEVEL (mcg/mL) |
TISSUE (FLUID) PLASMA (SERUM) RATIO |
| SKIN | 72-96 | 0.4 | 0.012 | 35 |
| LUNG | 72-96 | 4 | 0.012 | >100 |
| SPUTUM* | 2-4 | 1 | 0.64 | 2 |
| SPUTUM** | 10-12 | 2.9 | 0.1 | 30 |
| TONSIL*** | 9-18 | 4.5 | 0.03 | >100 |
| TONSIL*** | 180 | 0.9 | 0.006 | >100 |
| CERVIX**** | 19 | 2.8 | 0.04 | 70 |
max0-120max0-120DOSAGE AND ADMINISTRATION
maxmax0-24maxmax0-24
| Pharmacokinetic Parameter [mean (SD)] |
3-Day Regimen (20 mg/kg x 3 days) |
5-Day Regimen (12 mg/kg x 5 days) |
| n |
11 |
17 |
| Cmax (mcg/mL) |
1.1 (0.4) |
0.5 (0.4) |
| Tmax (hr) |
2.7 (1.9) |
2.2 (0.8) |
| Auc0-24 mcg•hr/mL |
7.9 (2.9) |
3.9 (1.9) |
DOSAGE AND ADMINISTRATION
Drug-Drug Interactions
max PRECAUTIONS - Drug Interactions
| Co-administered Drug | Dose of Co-administered Drug |
Dose of Azithromycin |
n |
Ratio (with/without azithromycin) of Co-administered Drug Pharmacokinetic Parameters (90% CI); No Effect = 1 |
|
|---|---|---|---|---|---|
| |
|
|
|
Mean Cmax | Mean AUC |
| Atorvastatin | 10 mg/day x 8 days | 500 mg/day PO on days 6-8 | 12 |
0.83 (0.63 to 1.08) |
1.01 (0.81 to 1.25) |
| Carbamazepine | 200 mg/day x 2 days, then 200 mg BID x 18 days |
500 mg/day PO for days 16-18 | 7 |
0.97 (0.88 to 1.06) |
0.96 (0.88 to 1.06) |
| Cetirizine | 20 mg/day x 11 days | 500 mg PO on day 7, then 250 mg/day on days 8-11 | 14 |
1.03 (0.93 to 1.14) |
1.02 (0.92 to 1.13) |
| Didanosine | 200 mg PO BID x 21 days | 1,200 mg/day PO on days 8-21 | 6 |
1.44 (0.85 to 2.43) |
1.14 (0.83 to 1.57) |
| Efavirenz | 400 mg/day x 7 days | 600 mg PO on day 7 | 14 |
1.04* | 0.95* |
| Fluconazole | 200 mg PO single dose | 1,200 mg PO single dose | 18 |
1.04 (0.98 to 1.11) |
1.01 (0.97 to 1.05) |
| Indinavir | 800 mg TID x 5 days | 1,200 mg PO on day 5 | 18 |
0.96 (0.86 to 1.08) |
0.90 (0.81 to 1.00) |
| Midazolam | 15 mg PO on day 3 | 500 mg/day PO x 3 days | 12 |
1.27 (0.89 to 1.81) |
1.26 (1.01 to 1.56) |
| Nelfinavir | 750 mg TID x 11 days | 1,200 mg PO on day 9 | 14 |
0.90 (0.81 to 1.01) |
0.85 (0.78 to 0.93) |
| Rifabutin | 300 mg/day x 10 days | 500 mg PO on day 1, then 250 mg/day on days 2-10 | 6 |
See footnote below |
NA |
| Sildenafil |
100 mg on days 1 and 4 | 500 mg/day PO x 3 days | 12 |
1.16 (0.86 to 1.57) |
0.92 (0.75 to 1.12) |
| Theophylline | 4 mg/kg IV on days 1, 11, 25 | 500 mg PO on day 7, 250 mg/day on days 8-11 | 10 |
1.19 (1.02 to 1.40) |
1.02 (0.86 to 1.22) |
| Theophylline | 300 mg PO BID x 15 days | 500 mg PO on day 6, then 250 mg/day on days 7-10 | 8 |
1.09 (0.92 to 1.29) |
1.08 (0.89 to 1.31) |
| Triazolam | 0.125 mg on day 2 | 500 mg PO on day 1, then 250 mg/day on day 2 | 12 |
1.06* | 1.02* |
| Trimethoprim/ Sulfamethoxazole | 160 mg/800mg/day PO x 7 days | 1,200 mg PO on day 7 | 12 |
0.85 (0.75 to 0.97)/ |
0.87 (0.80 to 0.95/ 0.96 (0.88 to 1.03) |
| Zidovudine | 500 mg/day PO x 21 days | 600 mg/day PO x 14 days | 5 |
1.12 (0.42 to 3.02) |
0.94 (0.52 to 1.70) |
| Zidovudine | 500 mg/day PO x 21 days | 1,200 mg/day PO x 14 days | 4 |
1.31 (0.43 to 3.97) |
1.30 (0.69 to 2.43) |
PRECAUTIONS - Drug Interactions.
| Co-administered Drug |
Dose of Co-administered Drug |
Dose of Azithromycin |
n | Ratio (with/without co-administered drug) of Azithromycin Pharmacokinetic Parameters (90% CI); No Effect = 1 |
|
|---|---|---|---|---|---|
| |
|
|
|
Mean Cmax | Mean AUC |
| Efavirenz | 400 mg/day x 7 days | 600 mg PO on day 7 | 14 |
1.22 (1.04 to 1.42) |
0.92* |
| Fluconazole | 200 mg PO single dose | 1,200 mg PO single dose | 18 |
0.82 (0.66 to 1.02) |
1.07 (0.94 to1.22) |
| Nelfinavir | 750 mg TID x 11 days | 1,200 mg PO on day 9 | 14 |
2.36 (1.77 to 3.15) |
2.12 (1.80 to 2.50) |
| Rifabutin | 300 mg/day x 10 days | 500 mg PO on day 1, then 250 mg/day on days 2-10 | 6 |
See footnote below |
NA |
Microbiology:
in vitroIn vivo
in vitroINDICATIONS AND USAGE
Aerobic and facultative gram-positive microorganisms
Staphylococcus aureus
Streptococcus agalactiae
Streptococcus pneumoniae
Streptococcus pyogenes
Enterococcus faecalis
Aerobic and facultative gram-negative microorganisms
Haemophilus ducreyi
Haemophilus influenzae
Moraxella catarrhalis
Neisseria gonorrhoeae
“Other” microorganisms
Chlamydia pneumoniae
Chlamydia trachomatis
Mycoplasma pneumoniae
in vitro but their clinical significance is unknown.
Aerobic and facultative gram-positive microorganisms
Aerobic and facultative gram-negative microorganisms
Bordetella pertussis
Legionella pneumophila
Anaerobic microorganisms
Peptostreptococcus species
Prevotella bivia
“Other” microorganisms
Ureaplasma urealyticum
Susceptibility Testing Methods:
in vitro
Dilution techniques:
1,3
Diffusion techniques:
2,3
Table 1. Susceptibility Interpretive Criteria for Azithromycin
Susceptibility Test Result Interpretive Criteria
Minimum Inhibitory Disk Diffusion
Pathogen Concentrations (mcg/mL) (zone diameters in mm)
S I Ra S I Ra
Haemophilus
Staphylococcus aureus
S. pneumoniaeb
a
bS. pneumoniae
Neisseria gonorrhoeae
QUALITY CONTROL:
Table 2. Acceptable Quality Control Ranges for Azithromycin
QC Strain Minimum Inhibitory Disk Diffusion
Concentrations (mcg/mL) (zone diameters in mm)
Haemophilus influenzae
Staphylococcus aureus
Staphylococcus aureus
Streptococcus pneumoniae
AZITHROMYCIN INDICATIONS AND USAGE
WARNINGSAs recommended dosages, durations of therapy and applicable patient populations vary among these infections, please see DOSAGE AND ADMINISTRATION for specific dosing recommendations.Adults:
Acute bacterial exacerbations of chronic obstructive pulmonary diseaseHaemophilus influenzaeMoraxella catarrhalis or Streptococcus pneumoniae.Acute bacterial sinusitisHaemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae.
Community-acquired pneumonia Chlamydia pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae or Streptococcus pneumoniae in patients appropriate
NOTE: Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors such as any of the following:
patients with cystic fibrosis,
patients with nosocomially acquired infections,
patients with known or suspected bacteremia,
patients requiring hospitalization,
elderly or debilitated patients, or
patients with significant underlying health problems that may compromise their ability to respond to their illness (including immunodeficiency or functional asplenia).
Pharyngitis/tonsillitisStreptococcus pyogenes
Streptococcus pyogenesStreptococcus pyogenes
Uncomplicated skin and skin structure infectionsStaphylococcus aureus, Streptococcus pyogenes,Streptococcus agalactiae
Urethritis and cervicitisChlamydia trachomatisNeisseria gonorrhoeae.
Genital ulcer diseaseHaemophilus ducreyi
Pediatric Patients:
PRECAUTIONS—Pediatric Use CLINICAL STUDIES IN PEDIATRIC PATIENTSAcute otitis media Haemophilus influenzae, Moraxella catarrhalisStreptococcus pneumoniaeDOSAGE AND ADMINISTRATION
Community-acquired pneumoniaChlamydia pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniaeStreptococcus pneumoniae DOSAGE AND ADMINISTRATION.
NOTE: Azithromycin should not be used in pediatric patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors such as any of the following:
patients with cystic fibrosis,
patients with nosocomially acquired infections,
patients with known or suspected bacteremia,
patients requiring hospitalization, or
patients with significant underlying health problems that may compromise their ability to respond to their illness (including immunodeficiency or functional asplenia).
Pharyngitis/tonsillitis Streptococcus pyogenesDOSAGE AND ADMINISTRATION
Streptococcus pyogenes
AZITHROMYCIN CONTRAINDICATIONS
WARNINGS
CONTRAINDICATIONSrecurred soon thereafter in some patients without further azithromycin exposureIn the treatment of pneumonia, azithromycin has only been shown to be safe and effective in the treatment of community-acquired pneumonia due to Chlamydia pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae or Streptococcus pneumoniae in patients appropriate for oral therapy. Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors such as any of the following: patients with cystic fibrosis, patients with nosocomially acquired infections, patients with known or suspected bacteremia, patients requiring hospitalization, elderly or debilitated patients, or patients with significant underlying health problems that may compromise their ability to respond to their illness (including immunodeficiency or functional asplenia).
Clostridium difficile C. difficile.
C. difficileC. difficile
C. difficileC. difficile
PRECAUTIONS
General: CLINICAL PHARMACOLOGY - Special Populations - Renal Insufficiency.
Information for Patients:
Drug Interactions:
ADVERSE REACTIONSCLINICAL PHARMACOLOGY-Drug-Drug Interactions.
Laboratory Test Interactions:
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Pregnancy:
Nursing Mothers:
Pediatric Use:
CLINICAL PHARMACOLOGY, INDICATIONS AND USAGE,DOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATION
Chlamydia pneumoniaeMycoplasma pneumoniaeHaemophilus influenzaeStreptococcus pneumoniae
Studies evaluating the use of repeated courses of therapy have not been conducted.CLINICAL PHARMACOLOGYANIMAL TOXICOLOGY.
Geriatric Use:
CLINICAL PHARMACOLOGY.AZITHROMYCIN ADVERSE REACTIONS
DOSAGE AND ADMINISTRATION.CLINICAL STUDIES IN PEDIATRIC PATIENTS.Clinical:
Adults:Multiple-dose regimens:
Cardiovascular:
Gastrointestinal:
Genitourinary:
Nervous System:
General:
Allergic:
Single 1-gramdose regimen:
Single 2-gram dose regimen:
Pediatric Patients:
Single and Multiple-dose regimens:
DOSAGE AND ADMINISTRATIONCLINICAL STUDIES IN PEDIATRIC PATIENTS
| Dosage Regimen |
Diarrhea, % |
Abdominal Pain, % |
Vomiting, % |
Nausea, % |
Rash, % |
|---|---|---|---|---|---|
| 1-day | 4.3% | 1.4% | 4.9% | 1.0% |
1.0% |
| 3-day |
2.6% |
1.7% |
2.3% | 0.4% |
0.6% |
| 5-day | 1.8% | 1.2% | 1.1% | 0.5% | 0.4% |
| Dosage Regimen |
Diarrhea/Loose stools, % | Abdominal Pain, % |
Vomiting, % | Nausea, % | Rash, % |
|---|---|---|---|---|---|
| |
|
|
|
|
|
| 5-day |
5.8% |
1.9% |
1.9% |
1.9% |
1.6% |
| Dosage Regimen |
Diarrhea, % | Abdominal Pain, % |
Vomiting, % | Nausea, % | Rash, % | Headache% |
|---|---|---|---|---|---|---|
| |
|
|
|
|
|
|
| 5-day |
5.4% |
3.4% |
5.6% |
1.8% |
0.7% | 1.1% |
| |
|
|
|
|
|
|
Cardiovascular:
Gastrointestinal:
Hematologic and Lymphatic:
Nervous System:
General:
Allergic:
Respiratory:
Skin and Appendages:
Special Senses:
Post-Marketing Experience:
Allergic:
Cardiovascular:torsades de pointes
Gastrointestinal:
General:
Genitourinary:
Hematopoietic:
Liver/Biliary:
Nervous System:
Psychiatric:
Skin/Appendages:
Special Senses:
Laboratory Abnormalities:
Adults:Pediatric Patients:
One, Three and Five Day Regimens
33DOSAGE AND ADMINISTRATION
AZITHROMYCIN DOSAGE AND ADMINISTRATION
INDICATIONS AND USAGE CLINICAL PHARMACOLOGYAdults:
| Infection* | Recommended Dose/Duration of Therapy |
| Community-acquired pneumonia (mild severity) Pharyngitis/tonsillitis (second line therapy) Skin/skin structure (uncomplicated) |
500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5. |
| Acute bacterial exacerbations of chronic obstructive pulmonary disease (mild to moderate) | 500 mg QD x 3 days OR 500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5. |
| Acute bacterial sinusitis | 500 mg QD x 3 days |
| Genital ulcer disease (chancroid) | One single 1 gram dose |
| Non-gonoccocal urethritis and cervicitis | One single 1 gram dose |
| Gonococcal urethritis and cervicitis | One single 2 gram dose |
0-120CLINICAL PHARMACOLOGY, Special Populations, Renal Insufficiency.
CLINICAL PHARMACOLOGY, Special Populations, Hepatic Insufficiency.
CLINICAL PHARMACOLOGY, Special Populations.
Pediatric Patients:
Acute Otitis Media:
Acute bacterial Sinusitis:
Community-Acquired Pneumonia:
| OTITIS MEDIA AND COMMUNITY-ACQUIRED PNEUMONIA: (5-Day Regimen)* Dosing Calculated on 10 mg/kg/day Day 1 and 5 mg/kg/day Days 2 to 5. |
|
||||||
|---|---|---|---|---|---|---|---|
| Weight |
|
100 mg/5 mL |
|
200 mg/5 mL |
|
Total mL per Treatment Course | Total mg per Treatment Course |
| Kg | Lbs. | Day 1 | Days 2-5 |
Day 1 | Days 2-5 | |
|
| 5 |
11 |
2.5 mL (½ tsp) |
1.25 mL (¼ tsp) |
|
|
7.5 mL |
150 mg |
| 10 |
22 |
5 mL (1 tsp) |
2.5 mL (½ tsp) |
|
|
15 mL |
300 mg |
| 20 |
44 |
|
|
5 mL (1 tsp) | 2.5 mL (½ tsp) | 15 mL |
600 mg |
| 30 |
66 |
|
|
7.5 mL (1½ tsp) | 3.75 mL (3/4tsp) | 22.5 mL |
900 mg |
| 40 |
88 |
|
|
10 mL (2 tsp) |
5 mL (1tsp) |
30 mL |
1200 mg |
| 50 and above |
|
|
|
12.5 mL (2 ½ tsp) |
6.25 mL (1¼ tsp) | 37.5 mL |
1500 mg |
| OTITIS MEDIA AND ACUTE BACTERIAL SINUSITIS: (3-Day Regimen)* Dosing Calculated on 10 mg/kg/day Day 1. |
|
||||
|---|---|---|---|---|---|
| Weight |
|
100 mg/5 mL | 200 mg/5 mL | Total mL per Treatment Course |
Total mg per Treatment Course |
| Kg | Lbs. | Day 1-3 | Day 1-3 | |
|
| 5 |
11 |
2.5 mL (½ tsp) |
|
7.5 mL |
150 mg |
| 10 |
22 |
5 mL (1 tsp) |
|
15 mL |
300 mg |
| 20 |
44 |
|
5 mL (1 tsp) |
15 mL |
600 mg |
| 30 |
66 |
|
7.5 mL (1½ tsp) |
22.5 mL |
900 mg |
| 40 |
88 |
|
10 mL (2 tsp) |
30 mL |
1200 mg |
| 50 and above |
110 and above |
|
12.5 mL (2 ½ tsp ) |
37.5 mL |
1500 mg |
| OTITIS MEDIA : (1-Day Regimen) Dosing Calculated on 30 mg/kg as a single dose |
||||
|---|---|---|---|---|
| Weight |
|
200 mg/5 mL |
Total mL per Treatment course |
Total mg per Treatment course |
| Kg | Lbs. |
Day1 |
|
|
| 5 |
11 |
3.75 mL (3/4 tsp) |
3.75 mL |
150 mg |
| 10 |
22 |
7.5 mL (1½ tsp) |
7.5 mL |
300 mg |
| 20 |
44 |
15 mL (3 tsp) | 15 mL |
600 mg |
| 30 |
66 |
22.5 mL (4 ½ tsp) | 22.5 mL |
900 mg |
| 40 |
88 |
30 mL (6tsp) | 30 mL |
1200 mg |
| 50 and above |
110 and above |
37.5 mL (7½ tsp) | 37.5 mL |
1500 mg |
Pharyngitis/Tonsillitis:
PEDIATRIC DOSAGE GUIDELINES FOR PHARYNGITIS /TONSILLITIS
(Age 2 years and above, see PRECAUTIONS-Pediatric Use.)
Based on Body weight
| PHARYNGITIS/TONSILITIS: (5-Day Regimen) Dosing Calculated on 12 mg/kg/day for 5 days |
|||||
|---|---|---|---|---|---|
| Weight |
|
200mg/5mL |
Total mL per Treatment course |
Total mg per Treatment course |
|
| Kg | Lbs. |
Day 1-5 |
|
|
|
| 8 |
18 |
2.5 mL (½ tsp) |
12.5 mL |
500 mg |
|
| 17 |
37 |
5 mL (1 tsp) |
25 mL |
1000 mg |
|
| 25 |
55 |
7.5 mL (1 ½ tsp) | 37.5 mL |
1500 mg |
|
| 33 |
73 |
10 mL (2 tsp) | 50 mL |
2000 mg | |
| 40 |
88 |
12.5 mL (2 ½ tsp) | 62.5 mL |
2500 mg |
HOW SUPPLIED
CLINICAL STUDIES
(See INDICATIONS AND USAGE and Pediatric Use.)
Pediatric Patients
From the perspective of evaluating pediatric clinical trials, Days 11-14 were considered on-therapy evaluations because of the extended half-life of azithromycin. Day 11-14 data are provided for clinical guidance. Day 24-32 evaluations were considered the primary test of cure endpoint.
Acute Otitis Media
Safety and efficacy using azithromycin 30 mg/kg given over 5 days
Protocol 1
In a double-blind, controlled clinical study of acute otitis media performed in the United States, azithromycin (10 mg/kg on Day 1 followed by 5 mg/kg on Days 2-5) was compared to amoxicillin/clavulanate potassium (4:1). For the 553 patients who were evaluated for clinical efficacy, the clinical success rate (i.e., cure plus improvement) at the Day 11 visit was 88% for azithromycin and 88% for the control agent. For the 521 patients who were evaluated at the Day 30 visit, the clinical success rate was 73% for azithromycin and 71% for the control agent.
In the safety analysis of the above study, the incidence of treatment-related adverse events, primarily gastrointestinal, in all patients treated was 9% with azithromycin and 31% with the control agent. The most common side effects were diarrhea/loose stools (4% azithromycin vs. 20% control), vomiting (2% azithromycin vs. 7% control), and abdominal pain (2% azithromycin vs. 5% control).
Protocol 2
In a non-comparative clinical and microbiologic trial performed in the United States, where significant rates of beta-lactamase producing organisms (35%) were found, 131 patients were evaluable for clinical efficacy. The combined clinical success rate (i.e., cure and improvement) at the Day 11 visit was 84% for azithromycin. For the 122 patients who were evaluated at the Day 30 visit, the clinical success rate was 70% for azithromycin.
Microbiologic determinations were made at the pre-treatment visit. Microbiology was not reassessed at later visits. The following presumptive bacterial/clinical cure outcomes (i.e., clinical success) were obtained from the evaluable group
| |
|
|
| |
Day 11 Azithromycin |
Day 30 Azithromycin |
| S. pneumoniae
|
61/74 (82%) |
40/56 (71%) |
| H. influenzae | 43/54 (80%) |
30/47 (64%) |
| M. catarrhalis
|
28/35 (80%) |
19/26 (73%) |
| S. pyogenes
|
11/11 (100%) |
7/7 |
| Overall |
177/217 (82%) |
97/137 (73%) |
In the safety analysis of this study, the incidence of treatment-related adverse events, primarily gastrointestinal, in all patients treated was 9%. The most common side effect was diarrhea (4%).
Protocol 3
In another controlled comparative clinical and microbiologic study of otitis media performed in the United States, azithromycin was compared to amoxicillin/clavulanate potassium (4:1). This study utilized two of the same investigators as Protocol 2 (above), and these two investigators enrolled 90% of the patients in Protocol 3. For this reason, Protocol 3 was not considered to be an independent study. Significant rates of beta-lactamase producing organisms (20%) were found. Ninety-two (92) patients were evaluable for clinical and microbiologic efficacy. The combined clinical success rate (i.e., cure and improvement) of those patients with a baseline pathogen at the Day 11 visit was 88% for azithromycin vs. 100% for control; at the Day 30 visit, the clinical success rate was 82% for azithromycin vs. 80% for control.
Microbiologic determinations were made at the pre-treatment visit. Microbiology was not reassessed at later visits. At the Day 11 and Day 30 visits, the following presumptive bacterial/clinical cure outcomes (i.e., clinical success) were obtained from the evaluable group
| Day 11 |
Day 30 | ||||
|---|---|---|---|---|---|
|
|
Azithromycin |
Control |
Azithromycin |
Control |
|
| S. pneumoniae
|
25/29 (86%) |
26/26 (100%) |
22/28 (79%) |
18/22 (82%) |
|
| H. influenzae | 9/11 (82%) |
9/9 |
8/10 (80%) |
6/8 |
|
| M. catarrhalis | 7/7 |
5/5 |
5/5 |
2/3 |
|
| S. pyogenes | 2/2 |
5/5 |
2/2 |
4/4 |
|
| Overall |
43/49 (88%) |
45/45 (100%) |
37/45 (82%) |
30/37 (81%) |
|
| |
|
|
|
|
|
| |
|
|
|
|
|
In the safety analysis of the above study, the incidence of treatment-related adverse events, primarily gastrointestinal, in all patients treated was 4% with azithromycin and 31% with the control agent. The most common side effect was diarrhea/loose stools (2% azithromycin vs. 29% control).
Safety and efficacy using azithromycin 30 mg/kg given over 3 days
Protocol 4
In a double-blind, controlled, randomized clinical study of acute otitis media in pediatric patients from 6 months to 12 years of age, azithromycin (10 mg/kg per day for 3 days) was compared to amoxicillin/clavulanate potassium (7:1) in divided doses q12h for 10 days. Each patient received active drug and placebo matched for the comparator.
For the 366 patients who were evaluated for clinical efficacy at the Day 12 visit, the clinical success rate (i.e., cure plus improvement) was 83% for azithromycin and 88% for the control agent. For the 362 patients who were evaluated at the Day 24-28 visit, the clinical success rate was 74% for azithromycin and 69% for the control agent.
In the safety analysis of the above study, the incidence of treatment-related adverse events, primarily gastrointestinal, in all patients treated was 10.6% with azithromycin and 20% with the control agent. The most common side effects were diarrhea/loose stools (5.9% azithromycin vs. 14.6% control), vomiting (2.1% azithromycin vs. 1.1% control), and rash (0% azithromycin vs. 4.3% control).
Safety and efficacy using azithromycin 30 mg/kg given as a single dose
Protocol 5
A double blind, controlled, randomized trial was performed at nine clinical centers. Pediatric patients from 6 months to 12 years of age were randomized 1:1 to treatment with either azithromycin (given at 30 mg/kg as a single dose on Day 1) or amoxicillin/clavulanate potassium (7:1), divided q12h for 10 days. Each child received active drug, and placebo matched for the comparator.
Clinical response (Cure, Improvement, Failure) was evaluated at End of Therapy (Day 12-16) and Test of Cure (Day 28-32). Safety was evaluated throughout the trial for all treated subjects. For the 321 subjects who were evaluated at End of Treatment, the clinical success rate (cure plus improvement) was 87% for azithromycin, and 88% for the comparator. For the 305 subjects who were evaluated at Test of Cure, the clinical success rate was 75% for both azithromycin and the comparator.
In the safety analysis, the incidence of treatment-related adverse events, primarily gastrointestinal, was 16.8% with azithromycin, and 22.5% with the comparator. The most common side effects were diarrhea (6.4% with azithromycin vs. 12.7% with the comparator), vomiting (4% with each agent), rash (1.7% with azithromycin vs. 5.2% with the comparator) and nausea (1.7% with azithromycin vs. 1.2% with the comparator).
Protocol 6
In a non-comparative clinical and microbiological trial, 248 patients from 6 months to 12 years of age with documented acute otitis media were dosed with a single oral dose of azithromycin (30 mg/kg on Day 1).
For the 240 patients who were evaluable for clinical modified Intent-to-Treat (MITT) analysis, the clinical success rate (i.e., cure plus improvement) at Day 10 was 89% and for the 242 patients evaluable at Day 24-28, the clinical success rate (cure) was 85%
| |
Day 10 |
Day 24-28 |
| S. pneumoniae | 70/76 (92%) |
67/76 (88%) |
| H. influenzae | 30/42 (71%) |
28/44 (64%) |
| M. catarrhalis | 10/10 (100%) |
10/10 (100%) |
| Overall |
110/128 (86%) |
105/130 (81%) |
Pharyngitis/Tonsillitis
| Azithromycin vs. Penicillin V |
||
|---|---|---|
|
|
EFFICACY RESULTS |
|
| |
Day 14 |
Day 30 |
| Bacteriologic Eradication |
|
|
| Azithromycin |
323/340 (95%) |
255/330 (77%) |
| Penicillin V |
242/332 (73%) |
206/325 (63%) |
| Clinical Success (Cure plus improvement) |
|
|
| Azithromycin |
336/343 (98%) |
310/330 (94%) |
| Penicillin V |
284/338 (84%) |
241/325 (74%) |
| |
|
Adult Patients
Acute Bacterial Exacerbations of Chronic Obstructive Pulmonary Disease| Pathogen | Azithromycin (3 Days) |
Clarithromycin (10 Days) |
| S. pneumoniae | 29/32 (91%) |
21/27 (78%) |
| H. influenzae | 12/14 (86%) |
14/16 (88%) |
| M. catarrhalis | 11/12 (92%) |
12/15 (80%) |
Acute Bacterial Sinusitis
ADVERSE REACTIONS
| Pathogen | Azithromycin (500 mg per day for 3 Days) |
|
|---|---|---|
| |
Day 7 | Day 28 |
| S. pneumoniae
|
23/26 (88%) | 21/25 (84%) |
| H. influenzae | 28/32 (87%) | 24/32 (75%) |
| M. catarrhalis | 14/15 (93%) | 13/15 (87%) |
ANIMAL TOXICOLOGY
2maxmaxmaxmax222REFERENCES:
- National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically – Fifth Edition. Approved Standard NCCLS Document M7-A5, Vol. 20, No. 2 (ISBN 1-56238-394-9). NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898, January 2000.
- National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disk Susceptibility Tests - Seventh Edition. Approved Standard NCCLS Document M2-A7, Vol. 20, No. 1 (ISBN 1-56238-393-0). NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898, January 2000.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing – Eleventh Informational Supplement. NCCLS Document M100-S11, Vol. 21, No. 1 (ISBN 1-56238-426-0). NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898, January 2001.
Manufactured by
Wockhardt Limited
Mumbai, India
Distributed by:
Wockhardt USA LLC.
20 Waterview Blvd.
Parsippany, NJ 07054
USA
Distributed by:
MAJOR® PHARMACEUTICALS
Livonia, MI 48150
REFER TO PACKAGE LABEL FOR DISTRIBUTOR'S NDC NUMBER
Rev.131010
PRINCIPAL DISPALY PANEL AZITHROMYCIN TABLETS 250MG

AzithromycinAzithromycin TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||