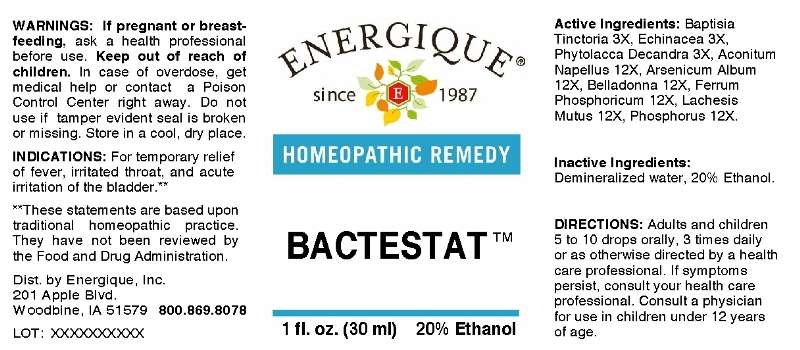
Bactestat
Energique, Inc.
Apotheca Company
Bactestat
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENTS: Baptisia tinctoria 3X, Echinacea 3X, Phytolacca decandra 3X, Aconitum napellus 12X, Arsenicum album 12x, Belladonna 12X, Ferrum phosphoricum 12X, Lachesis mutus 12x, Phosphorus 12X.
Purpose
INDICATIONS: For temporary relief of fever, irritated throat, and acute irritation of the bladder.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS: If pregnant or breast-feeding, seek advice of a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in a cool, dry place.
DIRECTIONS: Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist, consult your health care professional. Consult a physician for use in children under 12 years of age.
INACTIVE INGREDIENTS: Demineralized water, 20% Ethanol.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away.
Uses
INDICATIONS: For temporary relief of fever, irritated throat, and acute irritation of the bladder.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
Dist. by Energique, Inc.
201 Apple Blvd
Woodbine, IA 51579
800.869.8078
ENERGIQUE
since 1987
HOMEOPATHIC REMEDY
BACTESTAT
1 fl. oz. (30 ml)
BactestatBaptisia tinctoria, Echinacea, Phytolacca decandra, Aconitum napellus, Arsenicum album, Belladonna, Ferrum phosphoricum, Lachesis mutus, Phosphorus, LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||