Balsalazide Disodium
HIGHLIGHTS OF PRESCRIBING INFORMATION RECENT MAJOR CHANGESDrug Interactions (7) 2/2007Postmarketing Experience (6.2) 5/2008INDICATIONS AND USAGE Balsalazide is a locally acting aminosalicylate indicated for the treatment of mildly to moderately active ulcerative colitis in adults (1) Safety and effectiveness of balsalazide beyond 12 weeks in adults have not been established (1) DOSAGE AND ADMINISTRATION Adult dose is three 750 mg balsalazide disodium capsules 3 times a day (6.75 g/day) with or without food for 8 weeks. Some adult patients required treatment for up to 12 weeks. (2.1) Capsules may be swallowed whole or may be opened and sprinkled on applesauce, then chewed or swallowed immediately. (2.2, 12.3) DOSAGE FORMS AND STRENGTHSCapsules: 750 mg (3)CONTRAINDICATIONSPatients with hypersensitivity to salicylates or to any of the components of balsalazide disodium capsules or balsalazide metabolites. Hypersensitivity reactions may include, but are not limited to the following: anaphylaxis, bronchospasm, and skin reaction. (4)WARNINGS AND PRECAUTIONS Exacerbation of the symptoms of ulcerative colitis was reported in adult patients. Observe patients closely for worsening of these symptoms while on treatment. (5.1) Prolonged gastric retention of balsalazide may occur in patients with pyloric stenosis. (5.2) Side EffectsMost common adverse reactions in adults (incidence ≥3%) are headache, abdominal pain, diarrhea, nausea, vomiting, respiratory infection, and arthralgia. Adverse reactions in children were similar. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Apotex at 1-800-706-5575 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSIn an in vitro study using human liver microsomes, balsalazide and its metabolites were not shown to inhibit the major CYP enzymes evaluated (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5). (7)USE IN SPECIFIC POPULATIONSRenal impairment: Use balsalazide with caution in patients with a history of renal disease. (5.3)Pediatric: Pediatric use information is protected by marketing exclusivity. (8.4)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 BALSALAZIDE DISODIUM INDICATIONS AND USAGE
- 2 BALSALAZIDE DISODIUM DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 BALSALAZIDE DISODIUM CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 BALSALAZIDE DISODIUM ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 BALSALAZIDE DISODIUM DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Balsalazide disodium capsules, USP are indicated for the treatment of mildly to moderately active ulcerative colitis in adults. Safety and effectiveness of balsalazide beyond 12 weeks in adults have not been established.
2 DOSAGE AND ADMINISTRATION
2.1 Adult Dose
For treatment of active ulcerative colitis in adult patients, the usual dose is three 750 mg balsalazide disodium capsules to be taken 3 times a day (6.75 g per day) for up to 8 weeks. Some patients in the adult clinical trials required treatment for up to 12 weeks.
2.2 Administration Alternatives
Balsalazide disodium capsules may also be administered by carefully opening the capsule and sprinkling the capsule contents on applesauce. The entire drug/applesauce mixture should be swallowed immediately; the contents may be chewed, if necessary, since contents of balsalazide disodium capsules are NOT coated beads/granules. Patients should be instructed not to store any drug/applesauce mixture for future use.
If the capsules are opened for sprinkling, color variation of the powder inside the capsules ranges from orange to yellow and is expected due to color variation of the active pharmaceutical ingredient.
Teeth and/or tongue staining may occur in some patients who use balsalazide disodium capsules in sprinkle form with food.
3 DOSAGE FORMS AND STRENGTHS
Balsalazide is available as opaque white capsules containing 750 mg balsalazide disodium, imprinted with “APO B750” in red ink.
4 CONTRAINDICATIONS
Patients with hypersensitivity to salicylates or to any of the components of balsalazide disodium capsules or balsalazide metabolites. Hypersensitivity reactions may include, but are not limited to the following: anaphylaxis, bronchospasm, and skin reaction.
5 WARNINGS AND PRECAUTIONS
5.1 Exacerbations of Ulcerative Colitis
In the adult clinical trials, 3 out of 259 patients reported exacerbation of the symptoms of ulcerative colitis.
Observe patients closely for worsening of these symptoms while on treatment.
5.2 Pyloric Stenosis
Patients with pyloric stenosis may have prolonged gastric retention of balsalazide disodium capsules.
5.3 Renal
Renal toxicity has been observed in animals and patients given other mesalamine products. Therefore, caution should be exercised when administering balsalazide capsules to patients with known renal dysfunction or a history of renal disease. [See Nonclinical Toxicology (13.2)]
6 ADVERSE REACTIONS
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adult Ulcerative Colitis
During clinical development, 259 adult patients with active ulcerative colitis were exposed to 6.75 g/day balsalazide in 4 controlled trials.
In the 4 controlled clinical trials patients receiving a balsalazide dose of 6.75 g/day most frequently reported the following adverse reactions: headache (8%), abdominal pain (6%), diarrhea (5%), nausea (5%), vomiting (4%), respiratory infection (4%), and arthralgia (4%). Withdrawal from therapy due to adverse reactions was comparable among patients on balsalazide and placebo.
Adverse reactions reported by 1% or more of patients who participated in the four well controlled, Phase 3 trials are presented by treatment group (Table 1).
The number of placebo patients (35), however, is too small for valid comparisons. Some adverse reactions, such as abdominal pain, fatigue, and nausea were reported more frequently in women than in men. Abdominal pain, rectal bleeding, and anemia can be part of the clinical presentation of ulcerative colitis.
Table 1: Adverse Reactions Occurring in ≥ 1 % of Adult Balsalazide Patients in Controlled Trials*
| Adverse Reaction |
Balsalazide Capsules 6.75 g/day [N=259] |
Placebo [N=35] |
| Abdominal pain Diarrhea Arthralgia Rhinitis Insomnia Fatigue Flatulence Fever Dyspepsia Pharyngitis Coughing Anorexia Urinary tract infection Myalgia Flu-like disorder Dry mouth Cramps Constipation |
16 (6%) 14 (5%) 9 (4%) 6 (2%) 6 (2%) 6 (2%) 5 (2%) 5 (2%) 5 (2%) 4 (2%) 4 (2%) 4 (2%) 3 (1%) 3 (1%) 3 (1%) 3 (1%) 3 (1%) 3 (1%) |
1 (3%) 1 (3%) 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% |
*Adverse reactions occurring in at least 1 % of balsalazide Patients which were less frequent than placebo for the same event were not included in the table
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of balsalazide in clinical practice:
myocarditis, pericarditis, vasculitis, pruritus, pleural effusion, pneumonia (with and without eosinophilia), alveolitis, renal failure, interstitial nephritis, pancreatitis, and alopecia.
Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions have been chosen for inclusion due to a combination of seriousness, frequency of reporting, or potential causal connection to balsalazide.
Hepatic
Postmarketing adverse reactions of hepatotoxicity have been reported for products which contain (or are metabolized to) mesalamine, including elevated liver function tests (SGOT/AST, SGPT/ALT, GGT, LDH, alkaline phosphatase, bilirubin), jaundice, cholestatic jaundice, cirrhosis, hepatocellular damage including liver necrosis and liver failure. Some of these cases were fatal; however, no fatalities associated with these adverse reactions were reported in balsalazide clinical trials. One case of Kawasaki-like syndrome which included hepatic function changes was also reported, however, this adverse reaction was not reported in balsalazide clinical trials.
7 DRUG INTERACTIONS
In an in vitro study using human liver microsomes, balsalazide and its metabolites [5-aminosalicylic acid (5-ASA), N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA), 4-aminobenzoyl-β-alanine (4-ABA) and N-acetyl-4-aminobenzoyl-β–alanine (N-Ac-4-ABA)] were not shown to inhibit the major CYP enzymes evaluated (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5). Therefore, balsalazide and its metabolites are not expected to inhibit the metabolism of other drugs which are substrates of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A4/5.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. Reproduction studies were performed in rats and rabbits at oral doses up to 2 g/kg/day, 2.4 and 4.7 times the recommended human dose based on body surface area for the rat and rabbit, respectively, and revealed no evidence of impaired fertility or harm to the fetus due to balsalazide disodium. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
It is not known whether balsalazide disodium is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when balsalazide is administered to a nursing woman.
8.4 Pediatric Use
Pediatric use information is protected by marketing exclusivity.
10 OVERDOSAGE
No case of overdose has occurred with balsalazide. A 3-year-old boy is reported to have ingested 2 g of another mesalamine product. He was treated with ipecac and activated charcoal with no adverse reactions.
If an overdose occurs with balsalazide, treatment should be supportive, with particular attention to correction of electrolyte abnormalities.
11 DESCRIPTION
Each balsalazide disodium capsule, USP contains 750 mg of balsalazide disodium, a prodrug that is enzymatically cleaved in the colon to produce mesalamine (5-aminosalicylic acid or 5-ASA), an anti-inflammatory drug. Each capsule of balsalazide (750 mg) is equivalent to 267 mg of mesalamine. Balsalazide disodium has the chemical name (E)-5-[[-4-[[(2- carboxyethyl)amino]carbonyl] phenyl]azo]-2-hydroxybenzoic acid, disodium salt, dihydrate. Its structural formula is:
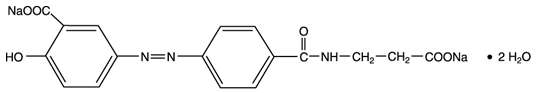
Molecular Weight: 437.32
Molecular Formula: C17H13N3O6Na2•2H2O
Balsalazide disodium is a stable, odorless orange to yellow microcrystalline powder. It is freely soluble in water and isotonic saline, sparingly soluble in methanol and ethanol, and practically insoluble in all other organic solvents.
Inactive Ingredients: Each hard gelatin capsule contains colloidal silicon dioxide, magnesium stearate and titanium dioxide. The capsule imprinting ink contains propylene glycol, strong ammonia solution, shellac and red iron oxide. The sodium content of each capsule is approximately 86 mg.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Balsalazide disodium is delivered intact to the colon where it is cleaved by bacterial azoreduction to release equimolar quantities of mesalamine, which is the therapeutically active portion of the molecule, and the 4-aminobenzoyl-β-alanine carrier moiety. The carrier moiety released when balsalazide disodium is cleaved is only minimally absorbed and is largely inert.
The mechanism of action of 5-ASA is unknown, but appears to be local to the colonic mucosa rather than systemic. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with chronic inflammatory bowel disease, and it is possible that 5-ASA diminishes inflammation by blocking production of arachidonic acid metabolites in the colon.
12.3 Pharmacokinetics
Balsalazide disodium capsules contain a powder of balsalazide disodium that is insoluble in acid and designed to be delivered to the colon as the intact prodrug. Upon reaching the colon, bacterial azoreductases cleave the compound to release 5-ASA, the therapeutically active portion of the molecule, and 4-aminobenzoyl-β-alanine. The 5-ASA is further metabolized to yield Nacetyl-5-aminosalicylic acid (N-Ac-5-ASA), a second key metabolite.
Absorption
The plasma pharmacokinetics of balsalazide and its key metabolites from a crossover study in healthy volunteers are summarized in Table 2. In this study, a single oral dose of balsalazide 2.25 g was administered to healthy volunteers as intact capsules (3 x 750 mg) under fasting conditions, as intact capsules (3 x 750 mg) after a high-fat meal, and unencapsulated (3 x 750 mg) as sprinkles on applesauce.
|
Fasting n = 17 |
High-Fat Meal n = 17 |
Sprinkled n = 17 |
|
| Cmax (mcg/mL) | |||
| Balsalazide | 0.51 ± 0.32 | 0.45 ± 0.39 | 0.21 ± 0.12 |
| 5-ASA | 0.22 ± 0.12 | 0.11 ± 0.136 | 0.29 ± 0.17 |
| N-Ac-5-ASA | 0.88 ± 0.39 | 0.64 ± 0.534 | 1.04 ± 0.57 |
| AUClast mcg·hr/mL) | |||
| Balsalazide | 1.35 ± 0.73 | 1.52 ± 1.01 | 0.87 ± 0.48 |
| 5-ASA | 2.59 ± 1.46 | 2.10 ± 2.58 | 2.99 ± 1.70 |
| N-Ac-5-ASA | 17.8 ± 8.14 | 17.7 ± 13.7 | 20.0 ± 11.4 |
| Tmax (h) | |||
| Balsalazide | 0.8 ± 0.85 | 1.2 ± 1.11 | 1.6 ± 0.44 |
| 5-ASA | 8.2 ± 1.98 | 22.0 ± 8.23 | 8.7 ± 1.99 |
| N-Ac-5-ASA | 9.9 ± 2.49 | 20.2 ± 8.94 | 10.8 ± 5.39 |
A relatively low systemic exposure was observed under all three administered conditions (fasting, fed with high-fat meal, sprinkled on applesauce), which reflects the variable, but minimal absorption of balsalazide disodium and its metabolites. The data indicate that both Cmax and AUClast were lower, while tmax was markedly prolonged, under fed (high-fat meal) compared to fasted conditions. Moreover, the data suggest that dosing balsalazide disodium as a sprinkle or as a capsule provides highly variable, but relatively similar mean pharmacokinetic parameter values. No inference can be made as to how the systemic exposure differences of balsalazide and its metabolites in this study might predict the clinical efficacy under different dosing conditions (i.e., fasted, fed with high-fat meal, or sprinkled on applesauce) since clinical efficacy after balsalazide disodium administration is presumed to be primarily due to the local effects of 5- ASA on the colonic mucosa.
In a separate study of adult patients with ulcerative colitis, who received balsalazide, 1.5 g twice daily, for over 1 year, systemic drug exposure, based on mean AUC values, was up to 60 times greater (0.008 mcg•hr/mL to 0.480 mcg•hr/mL) when compared to that obtained in healthy subjects who received the same dose.
Distribution
The binding of balsalazide to human plasma proteins was ≥99%.
Metabolism
The products of the azoreduction of this compound, 5-ASA and 4-aminobenzoyl-β-alanine, and their N-acetylated metabolites have been identified in plasma, urine and feces.
Elimination
Following single-dose administration of 2.25 g balsalazide (three 750 mg capsules) under fasting conditions in healthy subjects, mean urinary recovery of balsalazide, 5-ASA, and N-Ac-5-ASA was 0.20%, 0.22% and 10.2%, respectively.
In a multiple-dose study in healthy subjects receiving a balsalazide dose of two 750 mg capsules twice daily (3 g/day) for 10 days, mean urinary recovery of balsalazide, 5-ASA, and N-Ac-5-ASA was 0.1%, 0%, and 11.3%, respectively. During this study, subjects received their morning dose 0.5 hours after being fed a standard meal, and subjects received their evening dose 2 hours after being fed a standard meal.
In a study with 10 healthy volunteers, 65% of a single 2.25-gram dose of balsalazide was recovered as 5-ASA, 4-aminobenzoyl-β-alanine, and the N-acetylated metabolites in feces, while <1% of the dose was recovered as parent compound.
In a study that examined the disposition of balsalazide in patients who were taking 3 to 6 g of balsalazide daily for more than 1 year and who were in remission from ulcerative colitis, less than 1% of an oral dose was recovered as intact balsalazide in the urine. Less than 4% of the dose was recovered as 5-ASA, while virtually no 4-aminobenzoyl-β-alanine was detected in urine. The mean urinary recovery of N-Ac-5-ASA and N-acetyl-4-aminobenzoyl-β-alanine comprised <16% and <12% of the balsalazide dose, respectively. No fecal recovery studies were performed in this population.
All pharmacokinetic studies with balsalazide are characterized by large variability in the plasma concentration versus time profiles for balsalazide and its metabolites, thus half-life estimates of these analytes are indeterminate.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month rat (Sprague Dawley) carcinogenicity study, oral (dietary) balsalazide disodium at doses up to 2 g/kg/day was not tumorigenic. For a 50-kg person of average height this dose represents 2.4 times the recommended human dose on a body surface area basis. Balsalazide disodium was not genotoxic in the following in vitro or in vivo tests: Ames test, human lymphocyte chromosomal aberration test, and mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, or mouse micronucleus test. However, it was genotoxic in the in vitro Chinese hamster lung cell (CH V79/HGPRT) forward mutation test.
4-aminobenzoyl-β-alanine, a metabolite of balsalazide disodium, was not genotoxic in the Ames test and the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test but was positive in the human lymphocyte chromosomal aberration test. N-acetyl-4-aminobenzoyl-β-alanine, a conjugated metabolite of balsalazide disodium, was not genotoxic in Ames test, the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, or the human lymphocyte chromosomal aberration test. Balsalazide disodium at oral doses up to 2 g/kg/day, 2.4 times the recommended human dose based on body surface area, was found to have no effect on fertility and reproductive performance in rats.
13.2 Animal Toxicology
Renal Toxicity
In animal studies conducted at doses up to 2000 mg/kg (approximately 21 times the recommended 6.75 g/day dose on a mg/kg basis for a 70 kg person), balsalazide demonstrated no nephrotoxic effects in rats or dogs.
Overdosage
A single oral dose of balsalazide disodium at 5 g/kg or 4-aminobenzoyl-β-alanine, a metabolite of balsalazide disodium, at 1 g/kg was non-lethal in mice and rats. No symptoms of acute toxicity were seen at these doses.
14 CLINICAL STUDIES
14.1 Adult Studies
Two randomized, double-blind studies were conducted in adults. In the first trial, 103 patients with active mild-to-moderate ulcerative colitis with sigmoidoscopy findings of friable or spontaneously bleeding mucosa were randomized and treated with balsalazide 6.75 g/day or balsalazide 2.25 g/day. The primary efficacy endpoint was reduction of rectal bleeding and improvement of at least one of the other assessed symptoms (stool frequency, patient functional assessment, abdominal pain, sigmoidoscopic grade, and physician’s global assessment [PGA]). Outcome assessment for rectal bleeding at each interim period (week 2, 4, and 8) encompassed a 4-day period (96 hours). Results demonstrated a statistically significant difference between high and low doses of balsalazide (Figure 1).
Figure 1: Percentage of Patients Improved at 8 weeks
A second study, conducted in Europe, confirmed findings of symptomatic improvement
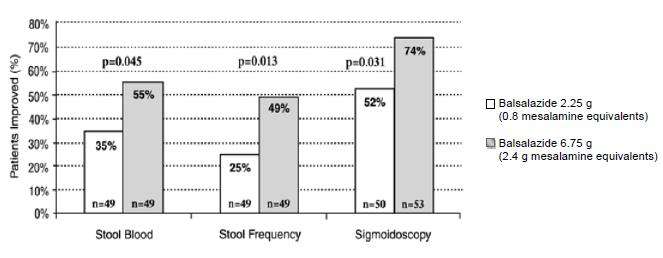
A second study, conducted in Europe, confirmed findings of symptomatic improvement.
16 HOW SUPPLIED/STORAGE AND HANDLING
Balsalazide Disodium Capsules, USP are available as white, opaque capsules imprinted “APO B750” in red ink.
Balsalazide Disodium Capsules, USP are supplied as follows:
Bottles of 30 (NDC 60505-2575-3)
Bottles of 280 (NDC 60505-2575-7)
Bottles of 350 (NDC 60505-2575-4)
Unit Dose Blister Packs of 10 strips of 10 capsules (NDC 60505-2575-0)
Storage
Store at 20º to 25ºC (68º to 77ºF); excursions permitted between 15º and 30ºC (59º and 86ºF).
See USP Controlled Room Temperature.
17 PATIENT COUNSELING INFORMATION
17.1 Important Precautions Regarding Balsalazide
- Instruct patients not to take balsalazide if they have a hypersensitivity to salicylates (e.g., aspirin).
- Patients should be instructed to contact their health care provider under the following circumstances:
- If they experience a worsening of their ulcerative colitis symptoms.
- If they are diagnosed with pyloric stenosis, because balsalazide disodium capsules may be slow to pass through their digestive tract.
- If they are diagnosed with renal dysfunction. Damage to the kidney has been observed in people given medications similar to balsalazide.
17.2 What Patients Should Know About Side Effects
- In adult clinical trials the most common adverse reactions were headache, abdominal pain, diarrhea, nausea, vomiting, respiratory infection, and arthralgia.
- Inform patients that this listing of adverse reactions is not complete and not all adverse reactions can be anticipated. If appropriate, a more comprehensive list of adverse reactions can be discussed with patients.
17.3 What Patients Should Know About Taking Balsalazide Disodium Capsules with Other Medication
- Based upon limited studies conducted in a test tube, balsalazide is not believed to interfere with other drugs by preventing how the liver functions. However, as the studies were limited in scope, you should always consult your doctor and discuss potential interactions prior to initiating any new drug.
APOTEX INC.
BALSALAZIDE DISODIUM CAPSULES, USP 750 mg
| Manufactured by | Manufactured for |
| Apotex Inc. | Apotex Corp. |
| Toronto, Ontario | Weston, Florida |
| Canada M9L 1T9 | USA 33326 |
Revised: February 2014
Revision: 5
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
PRINCIPAL DISPLAY PANEL - 750 mg BOTTLE LABEL
APOTEX CORP. NDC 60505-2575-3
Balsalazide Disodium Capsules
750 mg
Rx
280 count bottle

Balsalazide DisodiumBalsalazide Disodium CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||