Benicar
HIGHLIGHTS OF PRESCRIBING INFORMATION BOXED WARNING WARNING: FETAL TOXICITY See full prescribing information for complete boxed warning. When pregnancy is detected, discontinue Benicar as soon as possible (5.1). Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (5.1). RECENT MAJOR CHANGESWarnings and Precautions: Sprue-like enteropathy (5.5) 7/2013INDICATIONS AND USAGE Benicar is an angiotensin II receptor blocker (ARB) indicated for the treatment of hypertension, alone or with other antihypertensive agents, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions (1). DOSAGE AND ADMINISTRATION Indication Starting dose Dose Range Adult Hypertension (2.1) 20 mg once daily 20 - 40 mg once daily Pediatric Hypertension (6 - 16 years) (2.2) 20 to
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: FETAL TOXICITY
- 1 BENICAR INDICATIONS AND USAGE
- 2 BENICAR DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 BENICAR CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 BENICAR ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 BENICAR DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Benicar is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with Benicar.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
It may be used alone or in combination with other antihypertensive agents.
2 DOSAGE AND ADMINISTRATION
2.1 Adult Hypertension
Dosage must be individualized. The usual recommended starting dose of Benicar is 20 mg once daily when used as monotherapy in patients who are not volume-contracted. For patients requiring further reduction in blood pressure after 2 weeks of therapy, the dose of Benicar may be increased to 40 mg. Doses above 40 mg do not appear to have greater effect. Twice-daily dosing offers no advantage over the same total dose given once daily.
No initial dosage adjustment is recommended for elderly patients, for patients with moderate to marked renal impairment (creatinine clearance <40 mL/min) or with moderate to marked hepatic dysfunction [see Warnings and Precautions (5.4), Use in Specific Populations (8.5, 8.6, 8.7) and Clinical Pharmacology (12.3)]. For patients with possible depletion of intravascular volume (e.g., patients treated with diuretics, particularly those with impaired renal function), initiate Benicar under close medical supervision and give consideration to use of a lower starting dose [see Warnings and Precautions (5.3)].
Benicar may be administered with or without food.
If blood pressure is not controlled by Benicar alone, a diuretic may be added. Benicar may be administered with other antihypertensive agents.
2.2 Pediatric Hypertension (6 to 16 years of age)
Dosage must be individualized. For children who can swallow tablets, the usual recommended starting dose of Benicar is 10 mg once daily for patients who weigh 20 to <35 kg (44 to 77 lb), or 20 mg once daily for patients who weigh ≥35 kg. For patients requiring further reduction in blood pressure after 2 weeks of therapy, the dose of Benicar may be increased to a maximum of 20 mg once daily for patients who weigh <35 kg or 40 mg once daily for patients who weigh ≥35 kg.
Children <1 year of age must not receive Benicar for hypertension.
For children who cannot swallow tablets, the same dose can be given using an extemporaneous suspension as described below [see Clinical Pharmacology (12.3)]. Follow the suspension preparation instructions below to administer Benicar as a suspension.
Preparation of Suspension (for 200 mL of a 2 mg/mL suspension)
Add 50 mL of Purified Water to an amber polyethylene terephthalate (PET) bottle containing twenty Benicar 20 mg tablets and allow to stand for a minimum of 5 minutes. Shake the container for at least 1 minute and allow the suspension to stand for at least 1 minute. Repeat 1-minute shaking and 1-minute standing for four additional times. Add 100 mL of Ora-Sweet®* and 50 mL of Ora-Plus®* to the suspension and shake well for at least 1 minute. The suspension should be refrigerated at 2-8°C (36-46°F) and can be stored for up to 4 weeks. Shake the suspension well before each use and return promptly to the refrigerator.
* Ora-Sweet® and Ora-Plus® are registered trademarks of Paddock Laboratories, Inc.
3 DOSAGE FORMS AND STRENGTHS
- 5 mg yellow, round, film-coated, non-scored tablets debossed with Sankyo on one side and C12 on the other side
- 20 mg white, round, film-coated, non-scored tablets debossed with Sankyo on one side and C14 on the other side
- 40 mg white, oval-shaped, film-coated, non-scored tablets debossed with Sankyo on one side and C15 on the other side
4 CONTRAINDICATIONS
Do not co-administer aliskiren with Benicar in patients with diabetes [see Drug Interactions (7)].
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Benicar as soon as possible [see Use in specific Populations (8.1)].
5.2 Morbidity in Infants
Children <1 year of age must not receive Benicar for hypertension. Drugs that act directly on the renin-angiotensin aldosterone system (RAAS) can have effects on the development of immature kidneys [see Use in Specific Populations (8.4)].
5.3 Hypotension in Volume- or Salt-Depleted Patients
In patients with an activated renin-angiotensin aldosterone system, such as volume- and/or salt-depleted patients (e.g., those being treated with high doses of diuretics), symptomatic hypotension may be anticipated after initiation of treatment with Benicar. Initiate treatment under close medical supervision. If hypotension does occur, place the patient in the supine position and, if necessary, give an intravenous infusion of normal saline [see Dosage and Administration (2.1)]. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
5.4 Impaired Renal Function
As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals treated with Benicar. In patients whose renal function may depend upon the activity of the renin angiotensin-aldosterone system (e.g., patients with severe congestive heart failure), treatment with angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor antagonists has been associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death. Similar results may be anticipated in patients treated with Benicar [see Dosage and Administration (2.1), Drug Interactions (7), Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
In studies of ACE inhibitors in patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen (BUN) have been reported. There has been no long-term use of Benicar in patients with unilateral or bilateral renal artery stenosis, but similar results may be expected.
Severe, chronic diarrhea with substantial weight loss has been reported in patients taking olmesartan months to years after drug initiation. Intestinal biopsies of patients often demonstrated villous atrophy. If a patient develops these symptoms during treatment with olmesartan, exclude other etiologies. Consider discontinuation of Benicar in cases where no other etiology is identified.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adult Hypertension
Benicar has been evaluated for safety in more than 3825 patients/subjects, including more than 3275 patients treated for hypertension in controlled trials. This experience included about 900 patients treated for at least 6 months and more than 525 for at least 1 year. Treatment with Benicar was well tolerated, with an incidence of adverse reactions similar to placebo. Events generally were mild, transient and had no relationship to the dose of Benicar.
The overall frequency of adverse reactions was not dose-related. Analysis of gender, age and race groups demonstrated no differences between Benicar and placebo-treated patients. The rate of withdrawals due to adverse reactions in all trials of hypertensive patients was 2.4% (i.e., 79/3278) of patients treated with Benicar and 2.7% (i.e., 32/1179) of control patients. In placebo-controlled trials, the only adverse reaction that occurred in more than 1% of patients treated with Benicar and at a higher incidence versus placebo was dizziness (3% vs. 1%).
The following adverse reactions occurred in placebo-controlled clinical trials at an incidence of more than 1% of patients treated with Benicar, but also occurred at about the same or greater incidence in patients receiving placebo: back pain, bronchitis, creatine phosphokinase increased, diarrhea, headache, hematuria, hyperglycemia, hypertriglyceridemia, influenza-like symptoms, pharyngitis, rhinitis and sinusitis.
The incidence of cough was similar in placebo (0.7%) and Benicar (0.9%) patients.
Other potentially important adverse reactions that have been reported with an incidence of greater than 0.5%, whether or not attributed to treatment, in the more than 3100 hypertensive patients treated with Benicar monotherapy in controlled or open-label trials are listed below.
Body as a Whole: chest pain, peripheral edema
Central and Peripheral Nervous System: vertigo
Gastrointestinal: abdominal pain, dyspepsia, gastroenteritis, nausea
Heart Rate and Rhythm Disorders: tachycardia
Metabolic and Nutritional Disorders: hypercholesterolemia, hyperlipemia, hyperuricemia
Musculoskeletal: arthralgia, arthritis, myalgia
Skin and Appendages: rash
Facial edema was reported in five patients receiving Benicar. Angioedema has been reported with angiotensin II antagonists.
Laboratory Test Findings: In controlled clinical trials, clinically important changes in standard laboratory parameters were rarely associated with administration of Benicar.
Hemoglobin and Hematocrit: Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.3 g/dL and 0.3 volume percent, respectively) were observed.
Liver Function Tests: Elevations of liver enzymes and/or serum bilirubin were observed infrequently. Five patients (0.1%) assigned to Benicar and one patient (0.2%) assigned to placebo in clinical trials were withdrawn because of abnormal liver chemistries (transaminases or total bilirubin). Of the five Benicar patients, three had elevated transaminases, which were attributed to alcohol use, and one had a single elevated bilirubin value, which normalized while treatment continued.
Pediatric Hypertension
No relevant differences were identified between the adverse experience profile for pediatric patients aged 1 to16 years and that previously reported for adult patients.
6.2 Post-Marketing Experience
The following adverse reactions have been reported in post-marketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: Asthenia, angioedema, anaphylactic reactions
Gastrointestinal: Vomiting, sprue-like enteropathy [see Warnings and Precautions (5.5)]
Metabolic and Nutritional Disorders: Hyperkalemia
Musculoskeletal: Rhabdomyolysis
Urogenital System: Acute renal failure, increased blood creatinine levels
Skin and Appendages: Alopecia, pruritus, urticaria
7 DRUG INTERACTIONS
No significant drug interactions were reported in studies in which Benicar was co-administered with digoxin or warfarin in healthy volunteers.
The bioavailability of olmesartan was not significantly altered by the co-administration of antacids [Al(OH)3/Mg(OH)2].
Olmesartan medoxomil is not metabolized by the cytochrome P450 system and has no effects on P450 enzymes; thus, interactions with drugs that inhibit, induce, or are metabolized by those enzymes are not expected.
Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including olmesartan medoxomil, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving olmesartan medoxomil and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including olmesartan medoxomil may be attenuated by NSAIDs including selective COX-2 inhibitors.
Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function and electrolytes in patients on Benicar and other agents that affect the RAS.
Do not co-administer aliskiren with Benicar in patients with diabetes [see Contraindications (4)]. Avoid use of aliskiren with Benicar in patients with renal impairment (GFR <60 ml/min).
Colesevelam hydrochloride
Concurrent administration of bile acid sequestering agent colesevelam hydrochloride reduces the systemic exposure and peak plasma concentration of olmesartan. Administration of olmesartan at least 4 hours prior to colesevelam hydrochloride decreased the drug interaction effect. Consider administering olmesartan at least 4 hours before the colesevelam hydrochloride dose [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Benicar as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive
use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Benicar, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to Benicar for hypotension, oliguria, and hyperkalemia [see Use in Specific Populations (8.4)].
8.3 Nursing Mothers
It is not known whether olmesartan is excreted in human milk, but olmesartan is secreted at low concentration in the milk of lactating rats. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Neonates with a history of in utero exposure to Benicar:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
The antihypertensive effects of Benicar were evaluated in one randomized, double-blind clinical study in pediatric patients 1 to 16 years of age [see Clinical Studies (14.2)]. The pharmacokinetics of Benicar were evaluated in pediatric patients 1 to 16 years of age [see Clinical Pharmacology (12.3)]. Benicar was generally well tolerated in pediatric patients, and the adverse experience profile was similar to that described for adults.
Benicar has not been shown to be effective for hypertension in children <6 years of age.
Children <1 year of age must not receive Benicar for hypertension [see Warnings and Precautions (5.2)]. The renin-angiotensin aldosterone system (RAAS) plays a critical role in kidney development. RAAS blockade has been shown to lead to abnormal kidney development in very young mice. Administering drugs that act directly on the renin- angiotensin aldosterone system (RAAS) can alter normal renal development.
8.5 Geriatric Use
Of the total number of hypertensive patients receiving Benicar in clinical studies, more than 20% were 65 years of age and over, while more than 5% were 75 years of age and older. No overall differences in effectiveness or safety were observed between elderly patients and younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out [see Dosage and Administration (2.1) and Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
Increases in AUC0-∞ and Cmax were observed in patients with moderate hepatic impairment compared to those in matched controls, with an increase in AUC of about 60%. No initial dosage adjustment is recommended for patients with moderate to marked hepatic dysfunction [see Dosage and Administration (2.1) and Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Patients with renal insufficiency have elevated serum concentrations of olmesartan compared to subjects with normal renal function. After repeated dosing, the AUC was approximately tripled in patients with severe renal impairment (creatinine clearance <20 mL/min). No initial dosage adjustment is recommended for patients with moderate to marked renal impairment (creatinine clearance <40 mL/min) [see Dosage and Administration (2.1), Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
8.8 Black Patients
The antihypertensive effect of Benicar was smaller in black patients (usually a low renin population), as has been seen with ACE inhibitors, beta-blockers and other angiotensin receptor blockers.
10 OVERDOSAGE
Limited data are available related to overdosage in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could be encountered if parasympathetic (vagal) stimulation occurs. If symptomatic hypotension occurs, initiate supportive treatment. The dialyzability of olmesartan is unknown.
11 DESCRIPTION
Olmesartan medoxomil, a prodrug, is hydrolyzed to olmesartan during absorption from the gastrointestinal tract. Olmesartan is a selective AT1 subtype angiotensin II receptor antagonist.
Olmesartan medoxomil is described chemically as 2,3-dihydroxy-2-butenyl 4-(1 hydroxy-1-methylethyl)-2-propyl-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5 carboxylate, cyclic 2,3-carbonate.
Its empirical formula is C29H30N6O6 and its structural formula is:

Olmesartan medoxomil is a white to light yellowish-white powder or crystalline powder with a molecular weight of 558.59. It is practically insoluble in water and sparingly soluble in methanol. Benicar is available for oral use as film-coated tablets containing 5 mg, 20 mg, or 40 mg of olmesartan medoxomil and the following inactive ingredients: hydroxypropyl cellulose, hypromellose, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, talc, titanium dioxide, and (5 mg only) yellow iron oxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation and renal reabsorption of sodium. Olmesartan blocks the vasoconstrictor effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in vascular smooth muscle. Its action is, therefore, independent of the pathways for angiotensin II synthesis.
An AT2 receptor is found also in many tissues, but this receptor is not known to be associated with cardiovascular homeostasis. Olmesartan has more than a 12,500-fold greater affinity for the AT1 receptor than for the AT2 receptor.
Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is a mechanism of many drugs used to treat hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because olmesartan medoxomil does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and circulating angiotensin II levels do not overcome the effect of olmesartan on blood pressure.
12.2 Pharmacodynamics
Benicar doses of 2.5 mg to 40 mg inhibit the pressor effects of angiotensin I infusion. The duration of the inhibitory effect was related to dose, with doses of Benicar >40 mg giving >90% inhibition at 24 hours.
Plasma concentrations of angiotensin I and angiotensin II and plasma renin activity (PRA) increase after single and repeated administration of Benicar to healthy subjects and hypertensive patients. Repeated administration of up to 80 mg Benicar had minimal influence on aldosterone levels and no effect on serum potassium.
12.3 Pharmacokinetics
Absorption
Olmesartan medoxomil is rapidly and completely bioactivated by ester hydrolysis to olmesartan during absorption from the gastrointestinal tract.
Benicar tablets and the suspension formulation prepared from Benicar tablets are bioequivalent [see Dosage and Administration (2.2)].
The absolute bioavailability of olmesartan is approximately 26%. After oral administration, the peak plasma concentration (Cmax) of olmesartan is reached after 1 to 2 hours. Food does not affect the bioavailability of olmesartan.
Distribution
The volume of distribution of olmesartan is approximately 17 L. Olmesartan is highly bound to plasma proteins (99%) and does not penetrate red blood cells. The protein binding is constant at plasma olmesartan concentrations well above the range achieved with recommended doses.
In rats, olmesartan crossed the blood-brain barrier poorly, if at all. Olmesartan passed across the placental barrier in rats and was distributed to the fetus. Olmesartan was distributed to milk at low levels in rats.
Metabolism and Excretion
Following the rapid and complete conversion of olmesartan medoxomil to olmesartan during absorption, there is virtually no further metabolism of olmesartan. Total plasma clearance of olmesartan is 1.3 L/h, with a renal clearance of 0.6 L/h. Approximately 35% to 50% of the absorbed dose is recovered in urine while the remainder is eliminated in feces via the bile.
Olmesartan appears to be eliminated in a biphasic manner with a terminal elimination half-life of approximately 13 hours. Olmesartan shows linear pharmacokinetics following single oral doses of up to 320 mg and multiple oral doses of up to 80 mg. Steady-state levels of olmesartan are achieved within 3 to 5 days and no accumulation in plasma occurs with once-daily dosing.
Geriatric
The pharmacokinetics of olmesartan were studied in the elderly (≥65 years). Overall, maximum plasma concentrations of olmesartan were similar in young adults and the elderly. Modest accumulation of olmesartan was observed in the elderly with repeated dosing; AUCss, τ was 33% higher in elderly patients, corresponding to an approximate 30% reduction in CLR
[see Dosage and Administration (2.1) and Use in Specific Populations (8.5)].
Pediatric
The pharmacokinetics of olmesartan were studied in pediatric hypertensive patients aged 1 to16 years. The clearance of olmesartan in pediatric patients was similar to that in adult patients when adjusted by the body weight [see Use in Specific Populations (8.4)].
Olmesartan pharmacokinetics have not been investigated in pediatric patients less than 1 year of age [see Warnings and Precautions (5.2) and Use in Specific Populations (8.4)].
Gender
Minor differences were observed in the pharmacokinetics of olmesartan in women compared to men. AUC and Cmax were 10-15% higher in women than in men.
Hepatic Insufficiency
Increases in AUC0-∞ and Cmax were observed in patients with moderate hepatic impairment compared to those in matched controls, with an increase in AUC of about 60% [see Dosage and Administration (2.1) and Use in Specific Populations (8.6)].
Renal Insufficiency
In patients with renal insufficiency, serum concentrations of olmesartan were elevated compared to subjects with normal renal function. After repeated dosing, the AUC was approximately tripled in patients with severe renal impairment (creatinine clearance <20 mL/min). The pharmacokinetics of olmesartan in patients undergoing hemodialysis has not been studied [see Dosage and Administration (2.1), Warnings and Precautions (5.4) and Use in Specific Populations (8.7)].
Bile acid sequestering agent colesevelam
Concomitant administration of 40 mg olmesartan medoxomil and 3750 mg colesevelam hydrochloride in healthy subjects resulted in 28% reduction in Cmax and 39% reduction in AUC of olmesartan. Lesser effects, 4% and 15% reduction in Cmax and AUC respectively, were observed when olmesartan m edoxomil was administered 4 hours prior to colesevelam hydrochloride [see Drug Interactions (7)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Olmesartan medoxomil was not carcinogenic when administered by dietary administration to rats for up to 2 years. The highest dose tested (2000 mg/kg/day) was, on a mg/m2 basis, about 480 times the maximum recommended human dose (MRHD) of 40 mg/day. Two carcinogenicity studies conducted in mice, a 6-month gavage study in the p53 knockout mouse and a 6-month dietary administration study in the Hras2 transgenic mouse, at doses of up to 1000 mg/kg/day (about 120 times the MRHD), revealed no evidence of a carcinogenic effect of olmesartan medoxomil.
Both olmesartan medoxomil and olmesartan tested negative in the in vitro Syrian hamster embryo cell transformation assay and showed no evidence of genetic toxicity in the Ames (bacterial mutagenicity) test. However, both were shown to induce chromosomal aberrations in cultured cells in vitro (Chinese hamster lung) and tested positive for thymidine kinase mutations in the in vitro mouse lymphoma assay. Olmesartan medoxomil tested negative in vivo for mutations in the MutaMouse intestine and kidney and for clastogenicity in mouse bone marrow (micronucleus test) at oral doses of up to 2000 mg/kg (olmesartan not tested).
Fertility of rats was unaffected by administration of olmesartan medoxomil at dose levels as high as 1000 mg/kg/day (240 times the MRHD) in a study in which dosing was begun 2 (female) or 9 (male) weeks prior to mating.
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
No teratogenic effects were observed when olmesartan medoxomil was administered to pregnant rats at oral doses up to 1000 mg/kg/day (240 times the maximum recommended human dose [MRHD] of olmesartan medoxomil on a mg/m2 basis) or pregnant rabbits at oral doses up to 1 mg/kg/day (half the MRHD on a mg/m2 basis; higher doses could not be evaluated for effects on fetal development as they were lethal to the does). In rats, significant decreases in pup birth weight and weight gain were observed at doses ≥1.6 mg/kg/day, and delays in developmental milestones (delayed separation of ear auricula, eruption of lower incisors, appearance of abdominal hair, descent of testes, and separation of eyelids) and dose-dependent increases in the incidence of dilation of the renal pelvis were observed at doses ≥8 mg/kg/day. The no observed effect dose for developmental toxicity in rats is 0.3 mg/kg/day, about one-tenth the MRHD of 40 mg/day.
14 CLINICAL STUDIES
14.1 Adult Hypertension
The antihypertensive effects of Benicar have been demonstrated in seven placebo controlled studies at doses ranging from 2.5 mg to 80 mg for 6 to 12 weeks, each showing statistically significant reductions in peak and trough blood pressure. A total of 2693 patients (2145 Benicar; 548 placebo) with essential hypertension were studied. Benicar once daily lowered diastolic and systolic blood pressure. The response was dose-related, as shown in the following graph. A Benicar dose of 20 mg daily produces a trough sitting BP reduction over placebo of about 10/6 mmHg and a dose of 40 mg daily produces a trough sitting BP reduction over placebo of about 12/7 mmHg. Benicar doses greater than 40 mg had little additional effect. The onset of the antihypertensive effect occurred within 1 week and was largely manifest after 2 weeks.
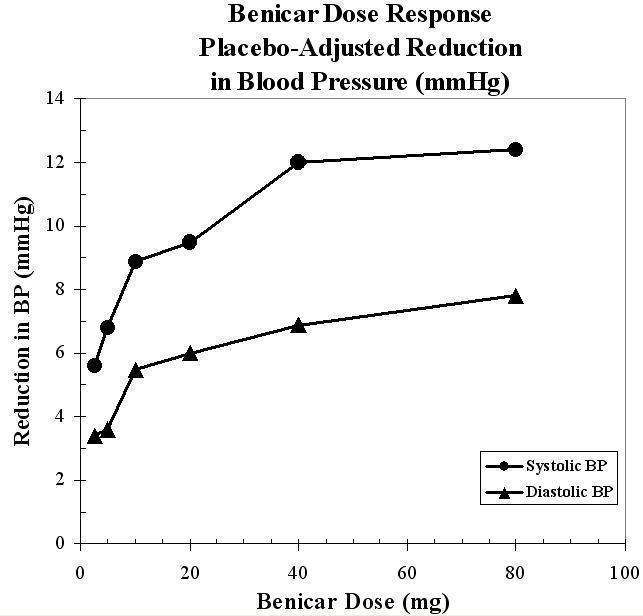
Data above are from seven placebo-controlled studies (2145 Benicar patients, 548 placebo patients). The blood pressure lowering effect was maintained throughout the 24-hour period with Benicar once daily, with trough-to-peak ratios for systolic and diastolic response between 60 and 80%.
The blood pressure lowering effect of Benicar, with and without hydrochlorothiazide, was maintained in patients treated for up to 1 year. There was no evidence of tachyphylaxis during long-term treatment with Benicar or rebound effect following abrupt withdrawal of olmesartan medoxomil after 1 year of treatment.
The antihypertensive effect of Benicar was similar in men and women and in patients older and younger than 65 years. The effect was smaller in black patients (usually a low renin population), as has been seen with ACE inhibitors, beta-blockers and other angiotensin receptor blockers. Benicar had an additional blood pressure lowering effect when added to hydrochlorothiazide.
There are no trials of Benicar demonstrating reductions in cardiovascular risk in patients with hypertension, but at least one pharmacologically similar drug has demonstrated such benefits.
14.2 Pediatric Hypertension
The antihypertensive effects of Benicar in the pediatric population were evaluated in a randomized, double-blind study involving 302 hypertensive patients aged 6 to 16 years. The study population consisted of an all black cohort of 112 patients and a mixed racial cohort of 190 patients, including 38 blacks. The etiology of the hypertension was predominantly essential hypertension (87% of the black cohort and 67% of the mixed cohort). Patients who weighed 20 to <35 kg were randomized to 2.5 or 20 mg of Benicar once daily and patients who weighed ≥35 kg were randomized to 5 or 40 mg of Benicar once daily. At the end of 3 weeks, patients were re-randomized to continuing Benicar or to taking placebo for up to 2 weeks. During the initial dose-response phase, Benicar significantly reduced both systolic and diastolic blood pressure in a weight-adjusted dose-dependent manner. Overall, the two dose levels of Benicar (low and high) significantly reduced systolic blood pressure by 6.6 and 11.9 mmHg from the baseline, respectively. These reductions in systolic blood pressure included both drug and placebo effect. During the randomized withdrawal to placebo phase, mean systolic blood pressure at trough was 3.2 mmHg lower and mean diastolic blood pressure at trough was 2.8 mmHg lower in patients continuing Benicar than in patients withdrawn to placebo. These differences were statistically different. As observed in adult populations, the blood pressure reductions were smaller in black patients.
In the same study, 59 patients aged 1 to 5 years who weighed ≥5 kg received 0.3 mg/kg of Benicar once daily for three weeks in an open label phase and then were randomized to receiving Benicar or placebo in a double-blind phase. At the end of the second week of withdrawal, the mean systolic/diastolic blood pressure at trough was 3/3 mmHg lower in the group randomized to Benicar; this difference in blood pressure was not statistically significant (95% C.I. -2 to 7/-1 to 7).
16 HOW SUPPLIED/STORAGE AND HANDLING
Benicar is supplied as yellow, round, film-coated, non-scored tablets containing 5 mg of olmesartan medoxomil, as white, round, film-coated, non-scored tablets containing 20 mg of olmesartan medoxomil, and as white, oval-shaped, film-coated, non-scored tablets containing 40 mg of olmesartan medoxomil. Tablets are debossed with Sankyo on one side and C12, C14, or C15 on the other side of the 5, 20, and 40 mg tablets, respectively.
Tablets are supplied as follows:
| 5 mg | 20 mg | 40 mg | |
| Bottle of 30 | NDC 65597-101-30 | NDC 65597-103-30 | NDC 65597-104-30 |
| Bottle of 90 | Not available | NDC 65597-103-90 | NDC 65597-104-90 |
| Blister 10 cards x 10 | Not available | NDC 65597-103-10 | NDC 65597-104-10 |
| Blister 1 card x 30 | Not available | NDC 65597-103-03 | NDC 65597-104-03 |
| Carton 6 cards x 30 | Not available | NDC 65597-103-06 | NDC 65597-104-06 |
Storage
Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Pregnancy: Female patients of childbearing age should be told about the consequences of exposure to Benicar during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
Manufactured for Daiichi Sankyo, Inc., Parsippany, New Jersey 07054
Rx Only
Copyright © Daiichi Sankyo, Inc. 2009. All rights reserved.
Package Label - Principal Display Panel – 5 mg 30 ct Bottle Label

Package Label - Principal Display Panel – 20 mg 30 ct Sleeve

Package Label - Principal Display Panel – 40 mg 100 ct Carton

Benicarolmesartan medoxomil TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Benicarolmesartan medoxomil TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Benicarolmesartan medoxomil TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||