BioGlo
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Product Facts
Each sterile strip is impregnated with 1 mg. of fluorescein sodium U.S.P.
INDICATIONS:
For staining the anterior segment of the eye when fitting contact lenses, in disclosing corneal injury and in applanation tonometry.
DIRECTIONS FOR USE:
To insure full fluorescence and patient comfort, the BioGlo impregnated tip should be moistened before application. One or two drops of sterile irrigating or saline solution should be used for this purpose. Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
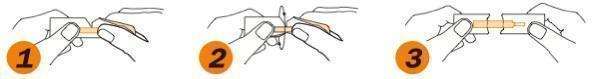
1. Hold package in middle and tear down toward sterile strip
2. Turn over and tear down from other side, taking care not to tear the sterile strip.
3. Grasp each end of package firmly as shown, without touching the sterile strip, and pull apart.
STORAGE:
Keep at room temperature.
NOTE:
For external use only. Contents may not be sterile if individual strips have been damaged or previously opened.
HOW SUPPLIED:
Dispenser carton containing 100 or 300 strips.
HUB Pharmaceuticals, LLC
Rancho Cucamonga, CA 91730
www.hubrx.com
Mfr. Lic. No. G/1197
European Representative:
Biovision Limited
Wayside, Tring Road, Wellhead, Dunstable, BEDS LU6 2JU, UK

17238-900-99: BioGlo Individual Pouch (above)

17238-900-11: Dispenser Carton containing 100 strips (above)

17238-900-30: Dispenser Carton containing 300 strips (above)
BioGloFluorescein Sodium STRIP
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||