Biscolax Laxative
Biscolax™ Laxative
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each suppository)
- Purpose
- Biscolax Laxative Uses
- Warnings
- Directions
- Biscolax Laxative Other information
- Inactive ingredient
- Questions?
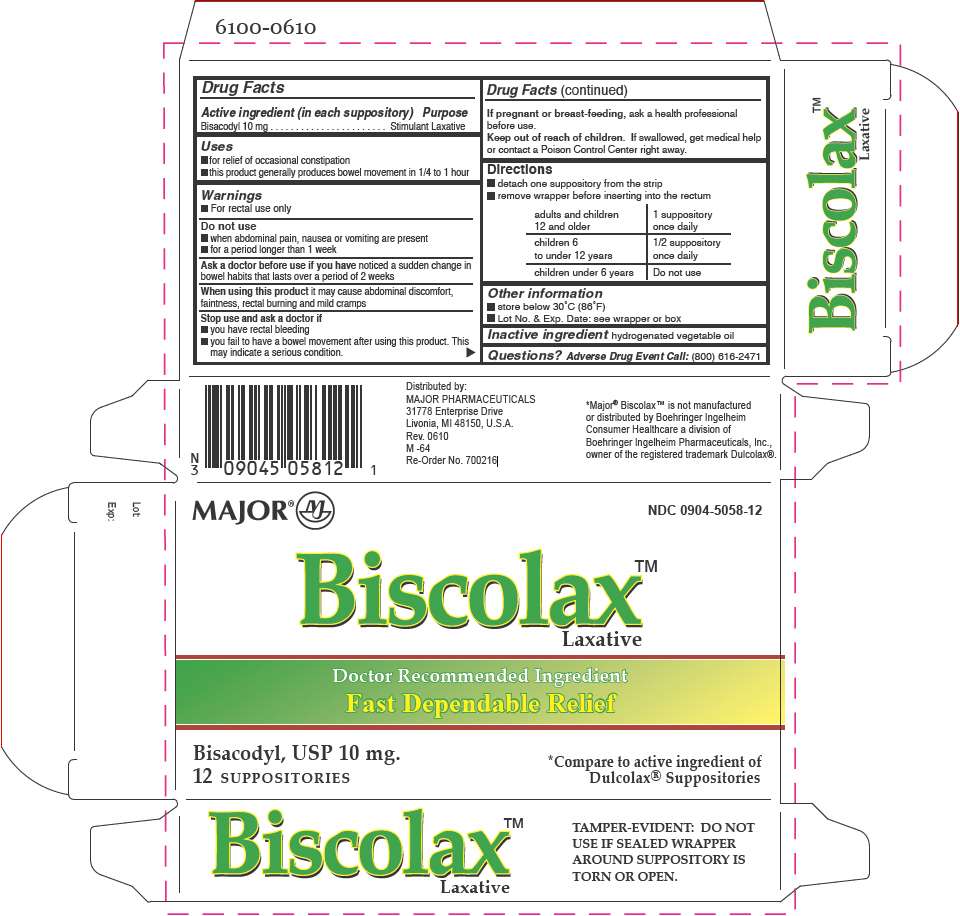
- PRINCIPAL DISPLAY PANEL - 12 Suppository Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient (in each suppository)
Bisacodyl 10 mg
Purpose
Stimulant Laxative
Biscolax Laxative Uses
- for relief of occasional constipation
- this product generally produces bowel movement in 1/4 to 1 hour
Warnings
- For rectal use only
Do not use
- when abdominal pain, nausea or vomiting are present
- for a period longer than 1 week
Ask a doctor before use if you have noticed a sudden change in bowel habits that lasts over a period of 2 weeks
When using this product it may cause abdominal discomfort, faintness, rectal burning and mild cramps
Stop use and ask a doctor if
- you have rectal bleeding
- you fail to have a bowel movement after using this product. This may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- detach one suppository from the strip
- remove wrapper before inserting into the rectum
| adults and children 12 and older | 1 suppository once daily |
| children 6 to under 12 years | 1/2 suppository once daily |
| children under 6 years | Do not use |
Biscolax Laxative Other information
- store below 30°C (86°F)
- Lot No. & Exp. Date: see wrapper or box
Inactive ingredient
hydrogenated vegetable oil
Questions?
Adverse Drug Event Call: (800) 616-2471
Distributed by:
MAJOR PHARMACEUTICALS
31778 Enterprise Drive
Livonia, MI 48150, U.S.A.
PRINCIPAL DISPLAY PANEL - 12 Suppository Carton
MAJOR ®
NDC 0904-5058-12
Biscolax™
Laxative
Doctor Recommended Ingredient
Fast Dependable Relief
Bisacodyl, USP 10 mg.
12 SUPPOSITORIES
*Compare to active ingredient of
Dulcolax® Suppositories
Biscolax LaxativeBisacodyl SUPPOSITORY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||