Bisoprolol Fumarate
Bisoprolol Fumarate Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- BISOPROLOL FUMARATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- BISOPROLOL FUMARATE INDICATIONS AND USAGE
- BISOPROLOL FUMARATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- BISOPROLOL FUMARATE ADVERSE REACTIONS
- OVERDOSAGE
- BISOPROLOL FUMARATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg (30 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (30 Tablet Bottle)
FULL PRESCRIBING INFORMATION
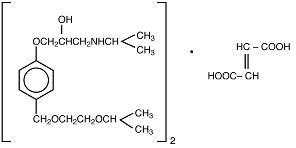
BISOPROLOL FUMARATE DESCRIPTION
1E183142444
CLINICAL PHARMACOLOGY
12
Pharmacokinetics and Metabolism
Pharmacodynamics
121
CLINICAL STUDIES
| Study A | Bisoprolol Fumarate | |||
|---|---|---|---|---|
| Placebo | 5 mg | 10 mg | 20 mg | |
|
a Observed total change from baseline minus placebo. |
||||
| n= |
61 |
61 |
61 |
61 |
| Total ΔBP (mm Hg) |
5.4/3.2 |
10.4/8 |
11.2/10.9 |
12.8/11.9 |
| Drug Effecta
|
- |
5/4.8 |
5.8/7.7 |
7.4/8.7 |
| Total ΔHR (bpm) |
0.5 |
7.2 |
8.7 |
11.3 |
| Drug Effecta
|
- |
6.7 |
8.2 |
10.8 |
|
Study B
|
Bisoprolol Fumarate
|
|||
|
Placebo
|
2.5 mg
|
10 mg
|
||
| n= |
56 |
59 |
62 |
|
| Total ΔBP (mm Hg) |
3/3.7 |
7.6/8.1 |
13.5/11.2 |
|
| Drug Effecta
|
- |
4.6/4.4 |
10.5/7.5 |
|
| Total ΔHR (bpm) |
1.6 |
3.8 |
10.7 |
|
| Drug Effecta
|
- |
2.2 |
9.1 |
|
BISOPROLOL FUMARATE INDICATIONS AND USAGE
BISOPROLOL FUMARATE CONTRAINDICATIONS
WARNINGS
Cardiac Failure
In Patients Without a History of Cardiac Failure
Abrupt Cessation of Therapy
Peripheral Vascular Disease
Bronchospastic Disease
PATIENTS WITH BRONCHOSPASTIC DISEASE SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS. Because of its relative beta1-selectivity, however, bisoprolol fumarate may be used with caution in patients with bronchospastic disease who do not respond to, or who cannot tolerate other antihypertensive treatment. Since beta1-selectivity is not absolute, the lowest possible dose of bisoprolol fumarate should be used, with therapy starting at 2.5 mg. A beta2 agonist (bronchodilator) should be made available.
Major Surgery
Diabetes and Hypoglycemia
1
Thyrotoxicosis
PRECAUTIONS
Impaired Renal or Hepatic Function
CLINICAL PHARMACOLOGY DOSAGE AND ADMINISTRATION
Drug Interactions
Risk of Anaphylactic Reaction:
Information for Patients
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitroin vivo
Pregnancy Category C
Nursing Mothers
Pediatric Use
Geriatric Use
BISOPROLOL FUMARATE ADVERSE REACTIONS
| Body System/Adverse Experience | All Adverse Experiences (%a) Bisoprolol Fumarate |
||
|---|---|---|---|
| Placebo (n=132) % |
5 to 20 mg (n=273) % |
2.5 to 40 mg (n=404) % |
|
|
a percentage of patients with event |
|||
| Skin |
|
|
|
| increased sweating |
1.5 |
0.7 |
1 |
| Musculoskeletal |
|
|
|
| arthralgia |
2.3 |
2.2 |
2.7 |
| Central Nervous System |
|
|
|
| dizziness |
3.8 |
2.9 |
3.5 |
| headache |
11.4 |
8.8 |
10.9 |
| hypoaesthesia |
0.8 |
1.1 |
1.5 |
| Autonomic Nervous System |
|
|
|
| dry mouth |
1.5 |
0.7 |
1.3 |
| Heart Rate/Rhythm |
|
|
|
| bradycardia |
0 |
0.4 |
0.5 |
| Psychiatric |
|
|
|
| vivid dreams |
0 |
0 |
0 |
| insomnia |
2.3 |
1.5 |
2.5 |
| depression |
0.8 |
0 |
0.2 |
| Gastrointestinal |
|
|
|
| diarrhea |
1.5 |
2.6 |
3.5 |
| nausea |
1.5 |
1.5 |
2.2 |
| vomiting |
0 |
1.1 |
1.5 |
| Respiratory |
|
|
|
| bronchospasm |
0 |
0 |
0 |
| cough |
4.5 |
2.6 |
2.5 |
| dyspnea |
0.8 |
1.1 |
1.5 |
| pharyngitis |
2.3 |
2.2 |
2.2 |
| rhinitis |
3 |
2.9 |
4 |
| sinusitis |
1.5 |
2.2 |
2.2 |
| URI |
3.8 |
4.8 |
5 |
| Body as a Whole |
|
|
|
| asthenia |
0 |
0.4 |
1.5 |
| chest pain |
0.8 |
1.1 |
1.5 |
| fatigue |
1.5 |
6.6 |
8.2 |
| edema (peripheral) |
3.8 |
3.7 |
3 |
Central Nervous System
unsteadinesssyncopesleep disturbances
Autonomic Nervous System
Cardiovascular
Psychiatric
Gastrointestinal
Musculoskeletal
arthralgia
Skin
psoriasisdermatitisangioedema, exfoliative dermatitis,
Special Senses
decreased hearing
Metabolic
Respiratory
Genitourinary
Peyronie‘s disease,
Hematologic
General
Central Nervous System
Allergic
Hematologic
Gastrointestinal
Miscellaneous
LABORATORY ABNORMALITIES
OVERDOSAGE
Bradycardia
Hypotension
Heart Block (second or third degree)
Congestive Heart Failure
Bronchospasm
Hypoglycemia
BISOPROLOL FUMARATE DOSAGE AND ADMINISTRATION
Bronchospastic Disease WARNINGS
Patients with Renal or Hepatic Impairment
Geriatric Patients
Geriatric Use in PRECAUTIONS
Pediatric Patients
HOW SUPPLIED
Bisoprolol Fumarate Tablets, USP 5 mg
Bisoprolol Fumarate Tablets, USP 10 mg
Store at
Dispense in tight, light-resistant containers as defined in the USP.
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg (30 Tablet Bottle)
NDC 65862-086-30
Bisoprolol Fumarate Tablets, USP
5 mg
Rx only 30 Tablets
AUROBINDO

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (30 Tablet Bottle)
NDC 65862-087-30
Bisoprolol Fumarate Tablets, USP
10 mg
Rx only 30 Tablets
AUROBINDO
Bisoprolol FumarateBisoprolol Fumarate TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Bisoprolol FumarateBisoprolol Fumarate TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!