BroveX PEB
MCR American Pharmaceuticals, Inc.
BrōveXPEB LIQUID
FULL PRESCRIBING INFORMATION: CONTENTS*
- BroveX PEB Uses
- Warnings
- Directions
- BroveX PEB Other information
- Inactive ingredients
- Question? Comments?
- PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
FULL PRESCRIBING INFORMATION
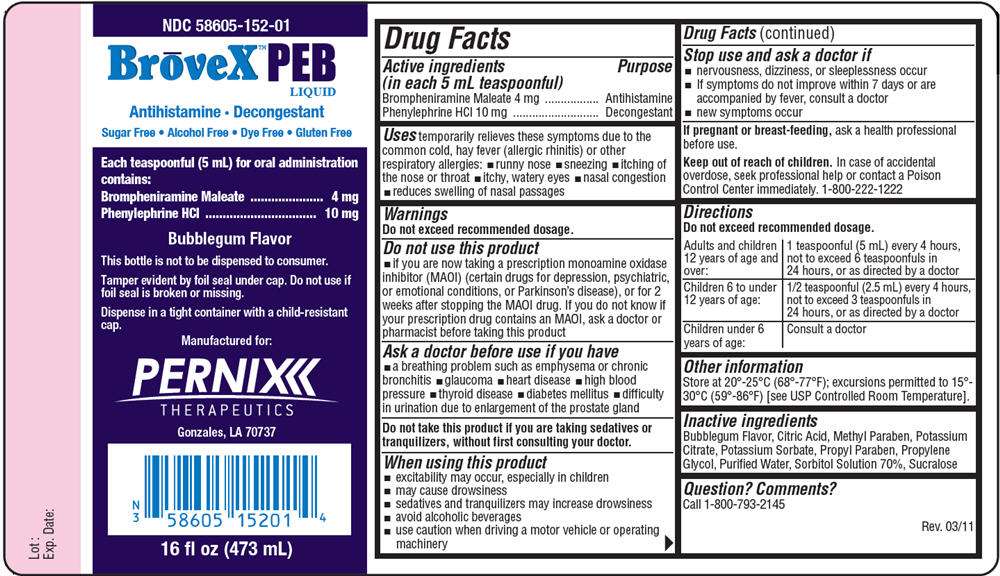
Drug Facts
Active ingredient
Purpose
|
Active ingredients (in each 5 mL teaspoonful) |
Purpose |
|---|---|
| Brompheniramine Maleate 4 mg | Antihistamine |
| Phenylephrine HCl 10 mg | Decongestant |
BroveX PEB Uses
temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- nasal congestion
- reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- heart disease
- high blood pressure
- thyroid disease
- diabetes mellitus
- difficulty in urination due to enlargement of the prostate gland
Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor.
When using this product
- excitability may occur, especially in children
- may cause drowsiness
- sedatives and tranquilizers may increase drowsiness
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- If symptoms do not improve within 7 days or are accompanied by fever, consult a doctor
- new symptoms occur
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of accidental overdose, seek professional help or contact a Poison Control Center immediately. 1-800-222-1222
Directions
Do not exceed recommended dosage.
| Adults and children 12 years of age and over: | 1 teaspoonful (5 mL) every 4 hours, not to exceed 6 teaspoonfuls in 24 hours, or as directed by a doctor |
| Children 6 to under 12 years of age: | 1/2 teaspoonful (2.5 mL) every 4 hours, not to exceed 3 teaspoonfuls in 24 hours, or as directed by a doctor |
| Children under 6 years of age: | Consult a doctor |
BroveX PEB Other information
Store at 20°-25°C (68°-77°F); excursions permitted to 15°- 30°C (59°-86°F) [see USP Controlled Room Temperature].
Inactive ingredients
Bubblegum Flavor, Citric Acid, Methyl Paraben, Potassium Citrate, Potassium Sorbate, Propyl Paraben, Propylene Glycol, Purified Water, Sorbitol Solution 70%, Sucralose
Question? Comments?
Call 1-800-793-2145
Rev. 03/11
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
NDC 58605-152-01
BrōveX™PEB
LIQUID
Antihistamine • Decongestant
Sugar Free • Alcohol Free • Dye Free • Gluten Free
| Each teaspoonful (5 mL) for oral administration contains: |
|
|---|---|
| Brompheniramine Maleate | 4 mg |
| Phenylephrine HCl | 10 mg |
Bubblegum Flavor
This bottle is not to be dispensed to consumer.
Tamper evident by foil seal under cap. Do not use if
foil seal is broken or missing.
Dispense in a tight container with a child-resistant
cap.
Manufactured for:
PERNIX
THERAPEUTICS
Gonzales, LA 70737
16 fl oz (473 mL)
BroveX PEBBrompheniramine Maleate and Phenylephrine Hydrochloride LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||