Buprenorphine Hydrochloride
BUPRENORPHINE HYDROCHLORIDE INJECTION
FULL PRESCRIBING INFORMATION
Rx Only
Buprenorphine hydrochloride injection is a narcotic under the Controlled Substances Act due to its chemical derivation from thebaine. Chemically, it is 17-(cyclopropylmethyl)-α-(1, 1-dimethylethyl)-4, 5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-6, 14-ethenomorphinan-7-methanol, hydrochloride [5α, 7α(S)].
Buprenorphine hydrochloride is a white powder, weakly acidic and with limited solubility in water. Buprenorphine hydrochloride injection is a clear, sterile, injectable agonist-antagonist analgesic intended for intravenous or intramuscular administration. Each mL of buprenorphine hydrochloride injection contains buprenorphine hydrochloride 0.324 mg (equivalent to buprenorphine 0.3 mg), anhydrous dextrose 50 mg, water for injection and HCI to adjust pH between 3.0 and 7.0.
Buprenorphine hydrochloride has the molecular formula, C29H41NO4HCI, and the following structure:
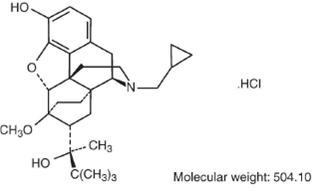
Buprenorphine hydrochloride injection is a parenteral opioid analgesic with 0.3 mg buprenorphine being approximately equivalent to 10 mg morphine sulfate in analgesic and respiratory depressant effects in adults. Pharmacological effects occur as soon as 15 minutes after intramuscular injection and persist for 6 hours or longer. Peak pharmacologic effects usually are observed at 1 hour. When used intravenously, the times to onset and peak effect are shortened.
The limits of sensitivity of available analytical methodology precluded demonstration of bioequivalence between intramuscular and intravenous routes of administration. In postoperative adults, pharmacokinetic studies have shown elimination half-lives ranging from 1.2 to 7.2 hours (mean 2.2 hours) after intravenous administration of 0.3 mg of buprenorphine. A single, ten-patient, pharmacokinetic study of doses of 3 mcg/kg in children (age 5 to 7 years) showed a high inter-patient variability, but suggests that the clearance of the drug may be higher in children than in adults. This is supported by at least one repeat-dose study in postoperative pain that showed an optimal inter-dose interval of 4 to 5 hours in pediatric patients as opposed to the recommended 6 to 8 hours in adults.
Buprenorphine, in common with morphine and other phenolic opioid analgesics, is metabolized by the liver and its clearance is related to hepatic blood flow. Studies in patients anesthetized with 0.5% halothane have shown that this anesthetic decreases hepatic blood flow by about 30%.
Buprenorphine hydrochloride exerts its analgesic effect via high affinity binding to µ subclass opiate receptors in the central nervous system. Although buprenorphine hydrochloride may be classified as a partial agonist, under the conditions of recommended use it behaves very much like classical µ agonists such as morphine. One unusual property of buprenorphine hydrochloride observed in in vitro studies is its very slow rate of dissociation from its receptor. This could account for its longer duration of action than morphine, the unpredictability of its reversal by opioid antagonists, and its low level of manifest physical dependence.
Buprenorphine demonstrates narcotic antagonist activity and has been shown to be equipotent with naloxone as an antagonist of morphine in the mouse tail flick test.
Buprenorphine hydrochloride injection may cause a decrease or, rarely, an increase in pulse rate and blood pressure in some patients.
Under usual conditions of use in adults, both buprenorphine hydrochloride injection and morphine show similar dose-related respiratory depressant effects. At adult therapeutic doses, buprenorphine hydrochloride injection (0.3 mg buprenorphine) can decrease respiratory rate in an equivalent manner to an equianalgesic dose of morphine (10 mg). (See WARNINGS.)
Buprenorphine hydrochloride injection is indicated for the relief of moderate to severe pain.
Buprenorphine hydrochloride injection should not be administered to patients who have been shown to be hypersensitive to the drug.
As with other potent opioids, clinically significant respiratory depression may occur within the recommended dose range in patients receiving therapeutic doses of buprenorphine. Buprenorphine hydrochloride should be used with caution in patients with compromised respiratory function (e.g., chronic obstructive pulmonary disease, cor pulmonale, decreased respiratory reserve, hypoxia, hypercapnia, or preexisting respiratory depression). Particular caution is advised if buprenorphine hydrochloride is administered to patients taking or recently receiving drugs with CNS/respiratory depressant effects. In patients with the physical and/or pharmacological risk factors above, the dose should be reduced by approximately one-half.
NALOXONE MAY NOT BE EFFECTIVE IN REVERSING THE RESPIRATORY DEPRESSION PRODUCED BY BUPRENORPHINE HYDROCHLORIDE INJECTION. THEREFORE, AS WITH OTHER POTENT OPIOIDS, THE PRIMARY MANAGEMENT OF OVERDOSE SHOULD BE THE REESTABLISHMENT OF ADEQUATE VENTILATION WITH MECHANICAL ASSISTANCE OF RESPIRATION, IF REQUIRED.
Patients receiving buprenorphine hydrochloride injection in the presence of other narcotic analgesics, general anesthetics, antihistamines, benzodiazepines, phenothiazines, other tranquilizers, sedative/hypnotics or other CNS depressants (including alcohol) may exhibit increased CNS depression. When such combined therapy is contemplated, it is particularly important that the dose of one or both agents be reduced.
Buprenorphine hydrochloride injection, like other potent analgesics, may itself elevate cerebrospinal fluid pressure and should be used with caution in head injury, intracranial lesions and other circumstances where cerebrospinal pressure may be increased. Buprenorphine hydrochloride injection can produce miosis and changes in the level of consciousness which may interfere with patient evaluation.
Buprenorphine hydrochloride injection may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery. Therefore, buprenorphine hydrochloride injection should be administered with caution to ambulatory patients who should be warned to avoid such hazards.
Because of the narcotic antagonist activity of buprenorphine hydrochloride injection, use in the physically dependent individual may result in withdrawal effects.
Buprenorphine hydrochloride injection should be administered with caution in the elderly, debilitated patients, in children and those with severe impairment of hepatic, pulmonary, or renal function; myxedema or hypothyroidism; adrenal cortical insufficiency (e.g., Addison's disease); CNS depression or coma; toxic psychoses; prostatic hypertrophy or urethral stricture; acute alcoholism; delirium tremens; or kyphoscoliosis.
Because buprenorphine hydrochloride injection is metabolized by the liver, the activity of buprenorphine hydrochloride injection may be increased and/or extended in those individuals with impaired hepatic function or those receiving other agents known to decrease hepatic clearance.
Buprenorphine hydrochloride injection has been shown to increase intracholedochal pressure to a similar degree as other opioid analgesics, and thus should be administered with caution to patients with dysfunction of the biliary tract.
The effects of buprenorphine hydrochloride injection, particularly drowsiness, may be potientiated by other centrally acting agents such as alcohol or benzodiazepines. It is particularly important that in these circumstances patients must not drive or operate machinery. Buprenorphine hydrochloride injection has some pharmacologic effects similar to morphine which in susceptible patients may lead to self-administration of the drug when pain no longer exists. Patients must not exceed the dosage of buprenorphine hydrochloride injection prescribed by their physician. Patients should be urged to consult their physician if other prescription medications are currently being used or are prescribed for future use.
Drug interactions common to other potent opioid analgesics also may occur with buprenorphine hydrochloride injection. Particular care should be taken when buprenorphine hydrochloride injection is used in combination with central nervous system depressant drugs (see WARNINGS). Although specific information is not presently available, caution should be exercised when buprenorphine hydrochloride injection is used in combination with MAO inhibitors. There have been reports of respiratory and cardiovascular collapse in patients who received therapeutic doses of diazepam and buprenorphine hydrochloride injection. A suspected interaction between buprenorphine hydrochloride injection and phenprocoumon resulting in purpura has been reported.
Since the metabolism of buprenorphine is mediated by the CYP3A4 isozyme, coadministration of drugs that inhibit CYP3A4 activity may cause decreased clearance of buprenorphine. Thus patients coadministered with inhibitors of CYP3A4 such as macrolide antibiotics (e.g., erythromycin), azole antifungal agents (e.g., ketoconazole), and protease inhibitors (e.g., ritanovir) while receiving buprenorphine hydrochloride injection should be carefully monitored and dosage adjustment made if warranted.
Cytochrome P450 inducers, such as rifampin, carbamazepine, and phenytoin, induce metabolism and as such may cause increased clearance of buprenorphine. Caution is advised when administering buprenorphine hydrochloride injection to patients receiving these medications and if necessary dose adjustments should be considered.
Carcinogenicity studies were conducted in Sprague-Dawley rats and CD-1 mice. Buprenorphine was administered in the diet at doses of 0.6, 5.5, and 56 mg/kg/day for 27 months in rats. These doses were approximately equivalent to 5.7, 52 and 534 times the recommended human dose (1.2 mg) on an mg/m2 body surface area basis. Statistically significant dose-related increases in testicular interstitial (Leydig's) cell tumors occurred, according to the trend test adjusted for survival. Pairwise comparison of the high dose against control failed to show statistical significance. In the mouse study, buprenorphine was administered in the diet at doses of 8, 50, and 100 mg/kg/day for 86 weeks. The high dose was approximately equivalent to 477 times the recommended human dose (1.2 mg) on a mg/m2 basis. Buprenorphine was not carcinogenic in mice.
Buprenorphine was studied in a series of tests. Results were negative in Chinese hamster bone marrow and spermatogonia cells, and negative in mouse lymphoma L5178Y assay. Results were equivocal in the Ames test: negative in studies in two laboratories, but positive in frame shift mutation at high dose (5 mg/plate) in a third study.
Reproduction studies of buprenorphine in rats demonstrated no evidence of impaired fertility at daily oral doses up to 80 mg/kg (approximately 763 times the recommended human daily dose of 1.2 mg on a mg/m2 basis) or up to 5 mg/kg I.M. or S.C. (approximately 48 times the recommended human daily dose of 1.2 mg on a mg/m2 basis)
Pregnancy Category C.
Buprenorphine was not teratogenic in rats or rabbits after I.M. or S.C. doses up to 5 mg/kg/day (approximately 48 and 95 times the recommended human daily dose of 1.2 mg on a mg/m2 basis), I.V. doses up to 0.8 mg/kg/day (approximately 8 times and 15 times the recommended human daily dose of 1.2 mg on a mg/m2 basis), or oral doses up to 160 mg/kg/day in rats (approximately 1525 times the recommended human daily dose of 1.2 mg on a mg/m2 basis) and 25 mg/kg/day in rabbits (approximately 475 times the recommended human daily dose of 1.2 mg on a mg/m2 basis). Significant increases in skeletal abnormalities (e.g., extra thoracic vertebra or thoraco-Iumbar ribs) were noted in rats after S.C. administration of 1 mg/kg/day and up (approximately 9.5 times the recommended human daily dose of 1.2 mg on a mg/m2 basis) and in rabbits after I.M. administration of 5 mg/kg/day (approximately 95 times the recommended human daily dose of 1.2 mg on a mg/m2 basis), but these increases were not statistically significant. Increases in skeletal abnormalities after oral administration were not observed in rats, and increases in rabbits (1 to 25 mg/kg/day) were not statistically significant.
There are no adequate and well-controlled studies in pregnant women. Buprenorphine hydrochloride injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The safety of buprenorphine hydrochloride injection given during labor and delivery has not been established.
An apparent lack of milk production during general reproduction studies with buprenorphine in rats caused decreased viability and lactation indices. Use of high doses of sublingual buprenorphine in pregnant women showed that buprenorphine passes into the mother's milk. Breast-feeding is therefore not advised in nursing mothers treated with buprenorphine hydrochloride injection.
The safety and effectiveness of buprenorphine hydrochloride injection have been established for children between 2 and 12 years of age. Use of buprenorphine hydrochloride injection in children is supported by evidence from adequate and well controlled trials of buprenorphine hydrochloride injection in adults, with additional data from studies of 960 children ranging in age from 9 months to 18 years of age. Data is available from a pharmacokinetic study, several controlled clinical trials, and several large post-marketing studies and case series. The available information provides reasonable evidence that buprenorphine hydrochloride injection may be used safely in children ranging from 2 to 12 years of age, and that it is of similar effectiveness in children as in adults.
The most frequent side effect in clinical studies involving 1,133 patients was sedation which occurred in approximately two-thirds of the patients. Although sedated, these patients could easily be aroused to an alert state.
| Nausea | Dizziness/Vertigo |
| Sweating | Headache |
| Hypotension | Nausea/Vomiting |
| Vomiting | Hypoventilation |
| Miosis |
CNS Effect: confusion, blurred vision, euphoria, weakness/fatigue, dry mouth, nervousness, depression, slurred speech, paresthesia.
Cardiovascular: hypertension, tachycardia, bradycardia.
Gastrointestinal: constipation.
Respiratory: dyspnea, cyanosis.
Dermatological: pruritus.
Ophthalmological: diplopia, visual abnormalities.
Miscellaneous: injection site reaction, urinary retention, dreaming, flushing/ warmth, chills/cold, tinnitus, conjunctivitis, Wenckebach block, and psychosis.
Other effects observed infrequently include malaise, hallucinations, depersonalization, coma, dyspepsia, flatulence, apnea, rash, amblyopia, tremor, and pallor.
The following reactions have been reported to occur rarely: loss of appetite, dysphoria/agitation, diarrhea, urticaria, and convulsions/lack of muscle coordination.
Allergic Reactions: Cases of acute and chronic hypersensitivity to buprenorphine have been reported both in clinical trials and in the post-marketing experience of buprenorphine hydrochloride injection and other buprenorphine-containing products. The most common signs and symptoms include rashes, hives, and pruritus. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. A history of hypersensitivity to buprenorphine is a contraindication to buprenorphine hydrochloride injection.
In the United Kingdom, buprenorphine hydrochloride was made available under monitored release regulation during the first year of sale, and yielded data from 1,736 physicians on 9,123 patients (17,120 administrations). Data on 240 children under the age of 18 years were included in this monitored release program. No important new adverse effects attributable to buprenorphine hydrochloride were observed.
Buprenorphine hydrochloride is a partial agonist of the morphine type, i.e., it has certain opioid properties which may lead to psychic dependence of the morphine type due to an opiate-like euphoric component of the drug. Direct dependence studies have shown little physical dependence upon withdrawal of the drug. However, caution should be used in prescribing to individuals who are known to be drug abusers or ex-narcotic addicts. The drug may not substitute in acutely dependent narcotic addicts due to its antagonist component and may induce withdrawal symptoms.
Clinical experience with buprenorphine hydrochloride injection overdosage has been insufficient to define the signs of this condition at this time. Although the antagonist activity of buprenorphine may become manifest at doses somewhat above the recommended therapeutic range, doses in the recommended therapeutic range may produce clinically significant respiratory depression in certain circumstances. (See WARNINGS.)
The respiratory and cardiac status of the patients should be monitored carefully. Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Doxapram, a respiratory stimulant, may be used.
NALOXONE MAY NOT BE EFFECTIVE IN REVERSING THE RESPIRATORY DEPRESSION PRODUCED BY BUPRENORPHINE HYDROCHLORIDE INJECTION. THEREFORE, AS WITH OTHER POTENT OPIOIDS, THE PRIMARY MANAGEMENT OF OVERDOSE SHOULD BE THE REESTABLISHMENT OF ADEQUATE VENTILATION WITH MECHANICAL ASSISTANCE OF RESPIRATION, IF REQUIRED.
The usual dosage for persons 13 years of age and over is 1 mL buprenorphine hydrochloride injection (0.3 mg buprenorphine) given by deep intramuscular or slow (over at least 2 minutes) intravenous injection at up to 6-hour intervals, as needed. Repeat once (up to 0.3 mg) if required, 30 to 60 minutes after initial dosage, giving consideration to previous dose pharmacokinetics, and thereafter only as needed. In high-risk patients (e.g., elderly, debilitated, presence of respiratory disease, etc.) and/or in patients where other CNS depressants are present, such as in the immediate postoperative period, the dose should be reduced by approximately one-half. Extra caution should be exercised with the intravenous route of administration, particularly with the initial dose.
Occasionally, it may be necessary to administer single doses of up to 0.6 mg to adults depending on the severity of the pain and the response of the patient. This dose should only be given I.M. and only to adult patients who are not in a high risk category (see WARNINGS and PRECAUTIONS). At this time, there are insufficient data to recommend single doses greater than 0.6 mg for long-term use.
Buprenorphine hydrochloride injection has been used in children 2 to 12 years of age at doses between 2 to 6 mcg/kg of body weight given every 4 to 6 hours. There is insufficient experience to recommend a dose in infants below the age of two years, single doses greater than 6 mcg/kg of body weight, or the use of a repeat or second dose at 30 to 60 minutes (such as is used in adults). Since there is some evidence that not all children clear buprenorphine faster than adults, fixed interval or "round-the-clock" dosing should not be undertaken until the proper inter-dose interval has been established by clinical observation of the child. Physicians should recognize that, as with adults, some pediatric patients may not need to be remedicated for 6 to 8 hours.
Buprenorphine hydrochloride injection is supplied in sealed vials and poses no known environmental risk to health care providers. Accidental dermal exposure should be treated by removal of any contaminated clothing and rinsing the affected area with water.
Buprenorphine hydrochloride injection is a potent narcotic, and like all drugs of this class has been associated with abuse and dependence among health care providers. To control the risk of diversion, it is recommended that measures appropriate to the health care setting be taken to provide rigid accounting, control of wastage, and restriction of access.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Buprenorphine hydrochloride injection, 0.3 mg (Base)/mL, is supplied in amber glass vials of 1 mL.
NDC 0517-0725-05; carton of 5 vials.
Avoid excessive heat (over 104°F or 40°C). Protect from prolonged exposure to light.
DISCARD UNUSED PORTION.
Store at 20° to 25°C (68° to 77°F) (See USP Controlled Room Temperature)
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
Revised December 2009
PRINCIPAL DISPLAY PANEL - 1 mL Label
NDC 0517-0725-01
BUPRENORPHINE
HYDROCHLORIDE
INJECTION
0.3 mg (base)/mL
1 mL VIAL
FOR IM OR IV USE
Protect From Light
Rx Only

PRINCIPAL DISPLAY PANEL - 1 mL Carton
NDC 0517-0725-05
CIII
5 x 1 mL Vials
BUPRENORPHINE HYDROCHLORIDE
INJECTION
0.3 mg (base)/mL
FOR INTRAMUSCULAR OR INTRAVENOUS USE
Rx Only

Buprenorphine HydrochlorideBuprenorphine Hydrochloride INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||