Calcium Chloride
Amphastar Pharmaceuticals, Inc.
CALCIUM CHLORIDE INJECTION, USP, 10%
FULL PRESCRIBING INFORMATION: CONTENTS*
- CALCIUM CHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CALCIUM CHLORIDE INDICATIONS AND USAGE
- CALCIUM CHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CALCIUM CHLORIDE ADVERSE REACTIONS
- CALCIUM CHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Syringe Assembly Directions
- PRINCIPAL DISPLAY PANEL - 10 mL Syringe Carton
- PRINCIPAL DISPLAY PANEL - 10 mL Syringe Carton
- PRINCIPAL DISPLAY PANEL - 10 mL Syringe Carton
FULL PRESCRIBING INFORMATION
13.6 mEq (1 g) per 10 mL
100 mg (1.36 mEq)/ mL
Osmolarity approximately 2050 mOsmol/L
CALCIUM CHLORIDE DESCRIPTION
Calcium Chloride Injection, USP, 10%, is a sterile aqueous solution containing, in each mL, 100 mg (1.36 mEq) calcium chloride. The pH of the solution may have been adjusted with hydrochloric acid and / or calcium hydroxide, when necessary. The air above the liquid in the individual containers has been displaced by flushing with nitrogen during the filling operation. The preparation contains no antimicrobial preservatives and is intended as a single-dose vial; once the unit is assembled and used, any remaining portion of the solution must be discarded with the entire unit.
Calcium Chloride, USP, contains two molecules of water of hydration and is chemically designated as CaCl2 • 2H20.
CLINICAL PHARMACOLOGY
Calcium is the fifth most abundant element in the body, the major fraction of which is found in the bony structure. Calcium plays important physiological roles; it is essential for the functional integrity of the nervous and muscular systems; it is necessary for normal cardiac function; and it is one of the factors involved in the mechanism of blood coagulation.
CALCIUM CHLORIDE INDICATIONS AND USAGE
Calcium Chloride Injection, USP, 10% is indicated:
-
-
-
-
-
CALCIUM CHLORIDE CONTRAINDICATIONS
Calcium chloride is contraindicated for cardiac resuscitation in the presence of ventricular fibrillation.
WARNINGS
Calcium chloride should be injected into a large vein very slowly, as it may cause peripheral vasodilatation and a cutaneous burning sensation. A moderate fall in blood pressure due to vasodilatation may attend the injection. Since calcium chloride is an acidifying salt, it is usually undesirable in the treatment of hypocalcemia or renal insufficiency.
PRECAUTIONS
General
Calcium Chloride Injection, USP, 10% is irritating to veins and must not be injected into tissues, since severe necrosis and sloughing may occur. Great care should be taken to avoid extravasation or accidental injection into perivascular tissues.
Solutions should be warmed to body temperature. Injections should be made slowly through a small needle into a large vein to minimize venous irritation and avoid undesirable reactions. It is particularly important to prevent a high concentration of calcium from reaching the heart because of the danger of cardiac syncope. If injected into the ventricular cavity in cardiac resuscitation care must be taken to avoid injection into the myocardial tissue.
Drug Interactions
Because of the danger involved in the simultaneous use of calcium salts and drugs of the digitalis group, a digitalized patient should not receive an intravenous injection of a calcium compound unless the indications are clearly defined.
Calcium salts should not generally be mixed with carbonates, phosphates, sulfates or tartrates in parenteral admixtures.
CALCIUM CHLORIDE ADVERSE REACTIONS
Rapid I.V. injection may cause the patient to complain of tingling sensations, a calcium taste, a sense of oppression or "heat wave."
Injections of calcium chloride are accompanied by peripheral vasodilation as well as a local "burning" sensation, and there may be a moderate fall in blood pressure.
CALCIUM CHLORIDE DOSAGE AND ADMINISTRATION
FOR INTRACARDIAC OR INTRAVENOUS USE ONLY
Please note that the optional STICK-GARD® Safety Needle, featured with stock number 2004 is interchangeable with a standard needle.
INJECT SLOWLY
Calcium Chloride Injection, USP, 10%, is administered only by slow intravenous injection (not to exceed 1 mL/min) and / or in cardiac resuscitation, by injection into the ventricular cavity. It must not be injected into the myocardium.
The usual precautions for intravenous therapy should be observed. If time permits, the solution should be warmed to body temperature. The injection should be halted if the patient complains of any discomfort; it may be resumed when symptoms disappear. Following injection, the patient should remain recumbent for a short time.
INTRACARDIAC USE
For cardiac resuscitation, inject into the ventricular cavity, not into the heart muscle.
Usual Adult Dosage
200 to 800 mg (2 to 8 mL) when injected into the ventricular cavity.
Pediatric Dosage
0.2 mL/kg of body weight.
INTRAVENOUS USE
Hypocalcemic Disorders
Usual Adult Dosage
500 mg to 1 g (5 to 10 mL) at intervals of 1 to 3 days, depending on the response of the patient and / or results of serum calcium determinations. Repeated injections may be required because of rapid excretion of calcium.
Pediatric Dosage
0.2 mL /kg of body weight. Maximum 1-10 mL/day.
Magnesium Intoxication
Initial Adult Dose
500 mg (5 mL) administered promptly and the patient observed for signs of recovery before further doses are given.
Hyperkalemic ECG Disturbances of Cardiac Function
Dosage should be adjusted by constant monitoring of ECG changes during administration.
HOW SUPPLIED
CALCIUM CHLORIDE INJECTION, USP, 10%
In unit-use packages containing a MIN-I-JET® disposable syringe.
| Stock No. 1004 (with 21 G. × 1 1/2" needle) |
NDC 0548-1004-00 | 10 mL |
In unit-use packages containing a disposable MIN-I-JET® Luer-Lock syringe with optional STICK-GARD® Safety Needle.
| Stock No. 2004 | NDC 0548-2004-00 | 10 mL |
Twenty-five unit-use packages per carton.
Manufactured under U.S. Pat. No. 4,834,716, Reissue No. 33,617, STICK-GARD® Safety Needle.
In unit-use packages containing a Luer-Jet™ Luer-Lock Prefilled Syringe.
| Stock No. 3304 | NDC 0548-3304-00 | 10 mL |
Ten unit-use packages per carton.
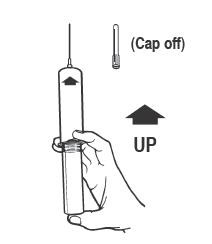
Syringe Assembly Directions
The MIN-I-JET® syringe with needle, illustrated below, is the basic unit upon which all the other syringe systems are built; slight adaptations and / or additional auxiliary parts create the other syringe systems. Assembly directions remain essentially the same.
USE ASEPTIC TECHNIQUE
Do not assemble until ready to use.

|

|
|
| Remove protective caps. Align vial such that the injector needle is centered on the stopper. |
Thread vial into injector 3 half turns or until needle penetrates stopper. |
Remove needle cap and expel air. |
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx Only
INTERNATIONAL MEDICATION SYSTEMS, LIMITED
So. El Monte, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company
Rev. 1-08
PRINCIPAL DISPLAY PANEL - 10 mL Syringe Carton
MIN-I-JET®
Prefilled Syringe
NDC 0548-1004-00
STOCK NO. 1004
Rx Only
CALCIUM CHLORIDE
INJ., USP, 10%
(100 mg/mL)
1.36 mEq/mL
1g
per
10 mL
FOR SLOW INTRAVENOUS USE ONLY
MIN-I-JET® 21 G. X 1 1/2" NEEDLE

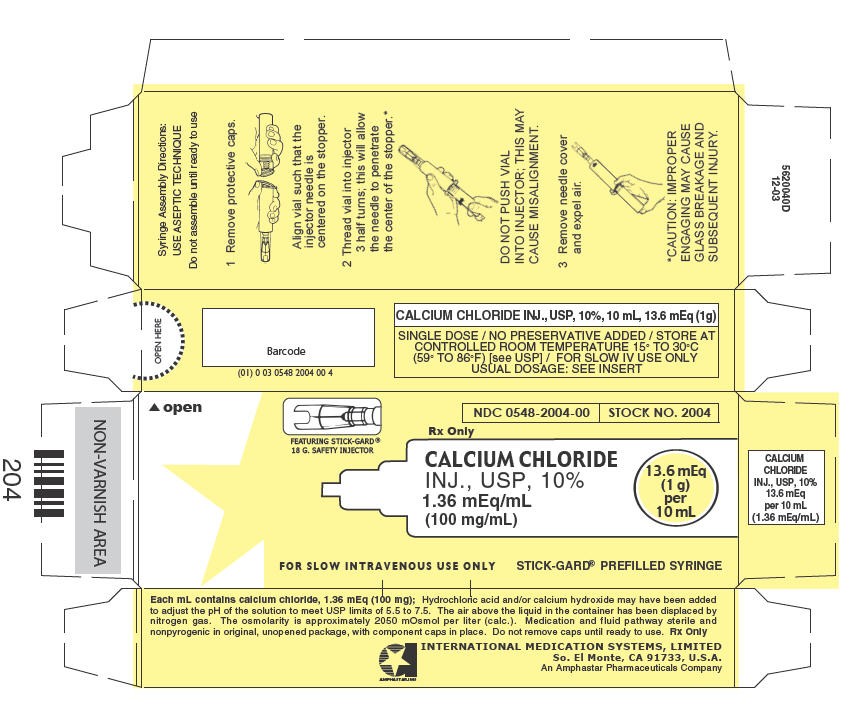
PRINCIPAL DISPLAY PANEL - 10 mL Syringe Carton
FEATURING STICK-GARD®
18 G. SAFETY INJECTOR
NDC 0548-2004-00
STOCK NO. 2004
Rx Only
CALCIUM CHLORIDE
INJ., USP, 10%
1.36 mEq/mL
(100 mg/mL)
13.6 mEq
(1 g)
per
10 mL
FOR SLOW INTRAVENOUS USE ONLY
STICK-GARD® PREFILLED SYRINGE
PRINCIPAL DISPLAY PANEL - 10 mL Syringe Carton
Luer-Lock Prefilled Syringe
NDC 0548-3304-00
STOCK NO. 3304
Rx Only
CALCIUM CHLORIDE
INJ., USP, 10%
1.36 mEq/ mL
(100 mg/ mL)
13.6 mEq
(1 g)
per
10 mL
FOR SLOW INTRAVENOUS USE
LUER-JET™ LUER-LOCK PREFILLED SYRINGE

Calcium ChlorideCalcium Chloride INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calcium ChlorideCalcium Chloride INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calcium ChlorideCalcium Chloride INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||