Carbamazepine
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use carisoprodol safely and effectively. See full prescribing information for carisoprodol tablets, USP. Carisoprodol Tablets, USP for Oral use CIVInitial U.S. Approval: 1959INDICATIONS AND USAGE(1) Should only be used for acute treatment periods up to two or three weeks (1) Not recommended in pediatric patients less than 16 years of age (8.4) DOSAGE AND ADMINISTRATION Recommended dose is 350 mg three times a day and at bedtime. (2) DOSAGE FORMS AND STRENGTHS(3)CONTRAINDICATIONS Acute intermittent porphyria (4) Hypersensitivity reactions to a carbamate such as meprobamate (4) WARNINGS AND PRECAUTIONS Due to sedative properties, may impair ability to perform hazardous tasks such as driving or operating machinery (5.1) Additive sedative effects when used with other CNS depressants including alcohol (5.1) Cases of Drug Dependence, Withdrawal, and Abuse (5.2) Seizures (5.3) Side Effects(6.1) To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) - additive sedative effects (5.1 and 7.1) RECENT MAJOR CHANGES(5.1)(5.2)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 CARBAMAZEPINE INDICATIONS AND USAGE
- 2 CARBAMAZEPINE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CARBAMAZEPINE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 CARBAMAZEPINE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 CARBAMAZEPINE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- Recent Major Changes
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 350 mg (100 Tablet Bottle)
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
see Dosage and Administration (2)
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Sedation
see ADVERSE REACTIONS (6.1)
5.2 Drug Dependence, Withdrawal, and Abuse
see Clinical Pharmacology (12.3)
5.3 Seizures
see Overdosage (10)
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
see Clinical Studies (14)
| Adverse Reaction |
Placebo (n=560) n (%) |
Carisoprodol 350 mg (n=279) n (%) |
|---|---|---|
| Drowsiness |
31 (6) |
47 (17) |
| Dizziness |
11 (2) |
19 (7) |
| Headache |
11 (2) |
9 (3) |
6.2 Postmarketing Experience
Cardiovascular: see Overdosage (10)
Central Nervous System: see Overdosage (10)
Gastrointestinal:
Hematologic:
7 DRUG INTERACTIONS
7.1 CNS Depressants
see Warnings and Precautions (5.1)
7.2 CYP2C19 Inhibitors and Inducers
see Clinical Pharmacology (12.3)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Pregnancy Category C.
Teratogenic effects:
Nonteratogenic effects: in-uteroin-utero
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Patients with Reduced CYP2C19 Activity
see Clinical Pharmacology (12.3)
9 DRUG ABUSE AND DEPENDENCE
see Warnings and Precautions (5.2)
In vitro
10 OVERDOSAGE
Treatment of Overdosage:
contact a Poison Control Center.
11 DESCRIPTION
122424
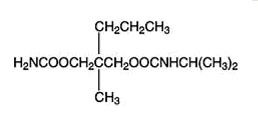
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
maxmax
| 350 mg Carisoprodol | |
|---|---|
| Carisoprodol
|
|
| Cmax (mcg/mL)
|
1.8 ± 1 |
| AUCinf (mcg*hr/mL)
|
7 ± 5 |
| Tmax (hr)
|
1.7 ± 0.8 |
| T1/2 (hr)
|
2 ± 0.5 |
| Meprobamate
|
|
| Cmax (mcg/mL)
|
2.5 ± 0.5 |
| AUCinf (mcg*hr/mL)
|
46 ± 9 |
| Tmax (hr)
|
4.5 ± 1.9 |
| T1/2 (hr)
|
9.6 ± 1.5 |
Absorption: max
Metabolism: Patients with Reduced CYP2C19 Activity
Elimination:
Gender:
Patients with Reduced CYP2C19 Activity:
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitro in vitro S. typhimurium in vivo
14 CLINICAL STUDIES
| Study | Parameter | Placebo | Carisoprodol 350 mg | |
|---|---|---|---|---|
| a The primary efficacy endpoints (Relief from Starting Backache and Global Impression of Change) were assessed by the patients on Study Day 3. These endpoints were scored on a 5-point rating scale from 0 (worst outcome) to 4 (best outcome). b Mean is the least squared mean and SE is the standard error of the mean. |
||||
| 1 |
Number of Patients
|
n=269 |
n=273 |
|
| Relief from Starting Backache, Mean (SE)b
|
1.4 (0.1) |
1.8 (0.1) |
||
| Difference between Carisoprodol and Placebo, Mean (SE)b (95% CI) |
0.4 (0.2, 0.6) |
|||
| Global Impression of Change, Mean (SE)b
|
1.9 (0.1) |
2.2 (0.1) |
||
| Difference between Carisoprodol and Placebo, Mean (SE)b (95% CI) |
0.3 (0.1, 0.4) |
16 HOW SUPPLIED/STORAGE AND HANDLING
Carisoprodol Tablets USP, 350 mg are white colored, round shaped, biconvex, uncoated tablets, identified with ‘D’ debossed on one side and ‘31’ on the other side.
Bottles of 60 NDC 33261-676-60
Store at controlled room temperature 20º to 25ºC (68º to 77ºF).
Dispense in tight container.
17 PATIENT COUNSELING INFORMATION
17.1 Sedation
see Warnings and Precautions (5.1)
17.2 Avoidance of Alcohol and Other CNS Depressants
see Warnings and Precautions (5.1)
17.3 Carisoprodol Should Only Be Used for Short-Term Treatment
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
Recent Major Changes
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 350 mg (100 Tablet Bottle)

CarbamazepineCarbamazepine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!