Carisoprodol
Sun Pharmaceutical Industries Limited
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use carisoprodol safely and effectively. See full prescribing information for carisoprodol tablets. Carisoprodol Tablets, USP C-IVfor Oral use Initial U.S. Approval: 1959 RECENT MAJOR CHANGES5.15.2INDICATIONS AND USAGE1 Should only be used for acute treatment periods up to two or three weeks (1) Not recommended in pediatric patients less than 16 years of age (8.4) DOSAGE AND ADMINISTRATION Recommended dose is 350 mg three times a day and at bedtime. (2) DOSAGE FORMS AND STRENGTHS3CONTRAINDICATIONS Acute intermittent porphyria (4) Hypersensitivity reactions to a carbamate such as meprobamate (4) WARNINGS AND PRECAUTIONS Due to sedative properties, may impair ability to perform hazardous tasks such as driving or operating machinery (5.1) Additive sedative effects when used with other CNS depressants including alcohol (5.1) Cases of Drug Dependence, Withdrawal, and Abuse (5.2) Seizures (5.3) Side Effects6.1To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) - additive sedative effects (5.1 and 7.1)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 CARISOPRODOL INDICATIONS AND USAGE
- 2 CARISOPRODOL DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CARISOPRODOL CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 CARISOPRODOL ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 CARISOPRODOL DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 350 MG
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
[see Dosage and Administration (2)]
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Sedation
see ADVERSE REACTIONS (6.1)
5.2 Drug Dependence, Withdrawal, and Abuse
see Clinical Pharmacology (12.3)
5.3 Seizures
) see Overdosage (10)
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
see Clinical Studies (14)
| Adverse Reaction | Placebo (n=560) n (%) |
Carisoprodol 350 mg (n=279) n (%) |
|---|---|---|
| Drowsiness |
31 (6) |
47 (17) |
| Dizziness |
11 (2) |
19 (7) |
| Headache |
11 (2) |
9 (3) |
6.2 Postmarketing Experience
Cardiovascular: see Overdosage (10)
Central Nervous System: see Overdosage (10)
Gastrointestinal:
Hematologic:
7 DRUG INTERACTIONS
7.1 CNS Depressants
see Warnings and Precautions (5.1)
7.2 CYP2C19 Inhibitors and Inducers
see Clinical Pharmacology (12.3)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Pregnancy Category C.
Teratogenic effects:
Nonteratogenic effects: in-uteroin-utero
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Patients with Reduced CYP2C19 Activity
see Clinical Pharmacology (12.3)
9 DRUG ABUSE AND DEPENDENCE
see Warnings and Precautions (5.2)
In vitro
10 OVERDOSAGE
Treatment of Overdosage:
contact a Poison Control Center.
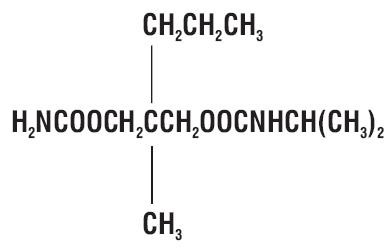
11 DESCRIPTION
122424
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
maxmax
|
350 mg
Carisoprodol
|
|
|
Carisoprodol
|
|
|
Cmax (mcg/mL)
|
1.8 ± 1 |
|
AUCinf (mcg*hr/mL)
|
7 ± 5 |
|
Tmax (hr)
|
1.7 ± 0.8 |
|
T1/2 (hr)
|
2 ± 0.5 |
|
Meprobamate
|
|
|
Cmax (mcg/mL)
|
2.5 ± 0.5 |
|
AUCinf (mcg*hr/mL)
|
46 ± 9 |
|
Tmax (hr)
|
4.5 ± 1.9 |
|
T1/2 (hr)
|
9.6 ± 1.5 |
Metabolism
Elimination
Gender:
Patients with Reduced CYP2C19 Activity:
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitro in vitro S. typhimurium in vivo
14 CLINICAL STUDIES
| Study | Parameter | Placebo | Carisoprodol 350 mg |
|---|---|---|---|
|
1
|
Number of Patients
|
n=269 |
n=273 |
Relief from Starting Backache, Mean (SE) |
1.4 (0.1) |
1.8 (0.1) |
|
Difference between Carisoprodol and Placebo, Mean (SE) |
0.4 (0.2, 0.6) |
||
Global Impression of Change, Mean (SE) |
1.9 (0.1) |
2.2 (0.1) |
|
Difference between Carisoprodol and Placebo, Mean (SE) |
0.3 (0.1, 0.4) |
16 HOW SUPPLIED/STORAGE AND HANDLING
Storage:
17 PATIENT COUNSELING INFORMATION
17.1 Sedation
see Warnings and Precautions (5.1)
17.2 Avoidance of Alcohol and Other CNS Depressants
see Warnings and Precautions (5.1)
17.3 Carisoprodol Tablets Should Only Be Used for Short-Term Treatment
To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 350 MG
NDC 62756-446-01
Carisoprodol Tablets, USP C-IV
350 mg
Rx only
30 TABLETS
SUN PHARMA

CarisoprodolCarisoprodol TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!