Carvedilol
REMEDYREPACK INC.
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
CARVEDILOL DESCRIPTION
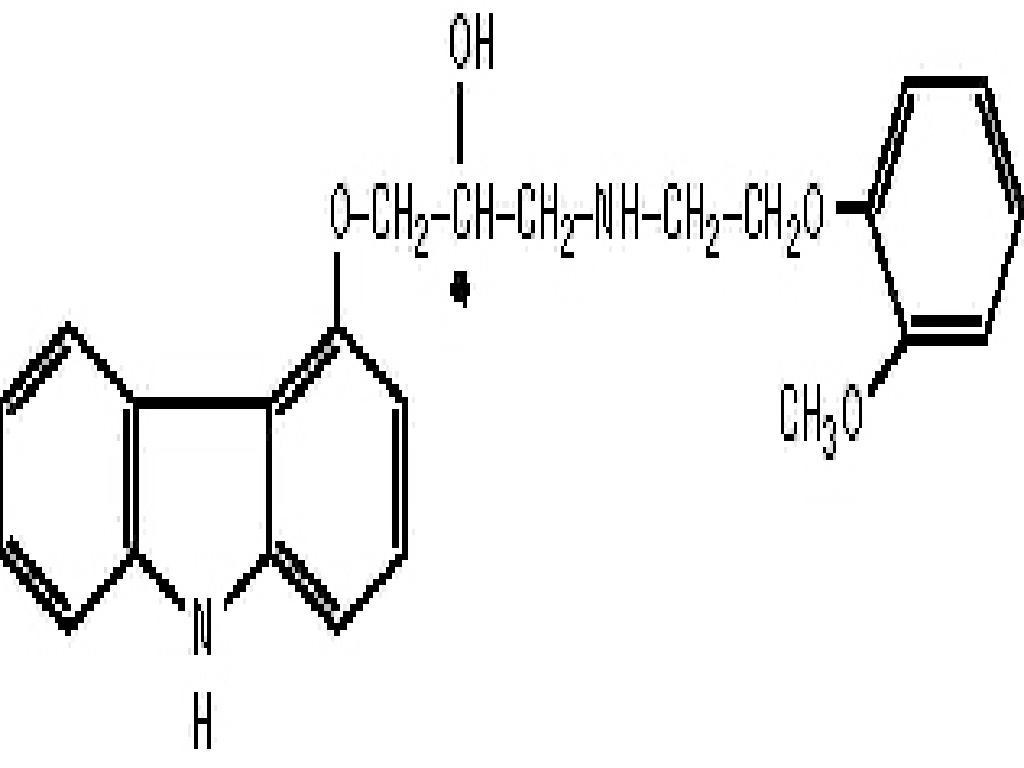
CLINICAL PHARMACOLOGY
Mechanism of ActionPHARMACODYNAMICS
PHARMACOKINETICS
USE IN SPECIFIC POPULATIONS
INDICATIONS & USAGE
CARVEDILOL CONTRAINDICATIONS
-
●Bronchial asthma or related bronchospastic conditions. Deaths from status asthmaticus have been reported following single doses of COREG.
-
●Second- or third-degree AV block
-
●Severe bradycardia (unless a permanent pacemaker is in place)
-
●Patients with cardiogenic shock or who have decompensated heart failure requiring the use of intravenous inotropic therapy. Such patients should first be weaned from intravenous therapy before initiating COREG.
-
●Patients with severe hepatic impairment
-
●Patients with a history of a serious hypersensitivity reaction (e.g., Stevens-Johnson syndrome, anaphylactic reaction, angioedema) to any component of this medication or other medications containing carvedilol.
WARNINGS AND PRECAUTIONS
DRUG INTERACTIONS
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
CARVEDILOL ADVERSE REACTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
DOSAGE FORMS & STRENGTHS
HOW SUPPLIED
INFORMATION FOR PATIENTS
-
●To treat patients with high blood pressure (hypertension)
-
●To treat patients who had a heart attack that worsened how well the heart pumps
-
●To treat patients with certain types of heart failure
-
●Are prone to asthma or other breathing problems
-
●Have a slow heartbeat or a heart that skips a beat (irregular heartbeat)
-
●Are allergic to any of the ingredients in COREG. The active ingredient is carvedilol. See the end of this leaflet for a list of all the ingredients in COREG.
-
●Have problems with blood flow in your feet and legs (peripheral vascular disease) COREG can make some of your symptoms worse.
-
●Have a condition called pheochromocytoma
-
●Have had severe allergic reactions
-
●Are pregnant or trying to become pregnant. It is not known if COREG is safe for your unborn baby. You and your doctor should talk about the best way to control your high blood pressure during pregnancy.
-
●Are breastfeeding. It is not known if COREG passes into your breast milk. You should not breastfeed while using COREG.
-
●Are scheduled for surgery and will be given anesthetic agents
-
●Are taking prescription or non-prescription medicines, vitamins, and herbal supplements. COREG and certain other medicines can affect each other and cause serious side effects. COREG may affect the way other medicines work. Also, other medicines may affect how well COREG works.
-
●Do not stop taking COREG and do not change the amount of COREG you take without talking to your doctor.
-
●Tell your doctor if you gain weight or have trouble breathing while taking COREG.
-
●If you miss a dose of COREG, take your dose as soon as you remember, unless it is time to take your next dose. Take your next dose at the usual time. Do not take 2 doses at the same time.
-
●If you take too much COREG, call your doctor or poison control center right away.
-
●Tiredness. If you feel tired or dizzy you should not drive, use machinery, or do anything that needs you to be alert.
-
●Changes in your blood sugar. If you have diabetes, tell your doctor if you have any changes in your blood sugar levels.
-
●COREG may hide some of the symptoms of low blood sugar, especially a fast heartbeat.
-
●COREG may mask the symptoms of hyperthyroidism (overactive thyroid).
-
●Worsening of severe allergic reactions.
-
●Rare but serious allergic reactions (including hives or swelling of the face, lips, tongue, and/or throat that may cause difficulty in breathing or swallowing) have happened in patients who were on COREG. These reactions can be life-threatening.
-
●Safely, throw away COREG that is out of date or no longer needed.
-
●Keep COREG and all medicines out of the reach of children.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Carvedilol
Carvedilol TABLET
Product Information
|
|
Product Type
|
Human prescription drug label |
Item Code (Source)
|
NDC:49349-032(NDC:55111-252) |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
Carvedilol CARVEDILOL |
|
3.125 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
yellow |
5 mm |
R;252 |
ROUND |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:49349-032-02 |
30 in 1 BLISTER PACK |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
ANDA |
ANDA076649 |
2011-08-02 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!


