Cefdinir
Cednifir Capsules
FULL PRESCRIBING INFORMATION: CONTENTS*
- 300 mg Rx only
- CEFDINIR DESCRIPTION
- CLINICAL PHARMACOLOGY
- CEFDINIR INDICATIONS AND USAGE
- Pediatric Patients:
- CEFDINIR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- Information for Patients:
- Drug Interactions:
- Drug/Laboratory Test Interactions
- Carcinogenesis, Mutagenesis, Impairment of Fertility:
- Pregnancy:
- Labor and Delivery:
- Nursing Mothers:
- Pediatric Use:
- ADVERSE EVENTS
- CEFDINIR DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- REFERENCES
FULL PRESCRIBING INFORMATION
300 mg Rx only
CEFDINIR DESCRIPTION
1413552
CLINICAL PHARMACOLOGY
maxmax
| Dose | C max (mcg/mL) | t max (hr) | AUC (mcg•hr/mL) |
| 300 mg | 1.60 | 2.9 | 7.05 |
|
|
(0.55) | (0.89) | (2.17) |
| 600 mg | 2.87 | 3.0 | 11.1 |
|
|
(1.01) | (0.66) | (3.87) |
| Dose | Cmax
(mcg/mL) |
tmax
(hr) |
AUC (mcg•hr/mL) |
| 7 mg/kg | 2.3 (0.65) |
2.2 (0.6) |
8.31 (2.5) |
| 14 mg/kg | 3.86 (0.62) |
1.8 (0.4) |
13.4 (2.64) |
areaarea
max(0-∞)
½ Special Populations: Patients with Renal Insufficiency
DOSAGE AND ADMINISTRATION
crcrmax½crmax½ DOSAGE AND ADMINISTRATION
½ DOSAGE AND ADMINISTRATION
max½ Patients with Renal Insufficiency
in vitro INDICATIONS AND USAGE
Staphylococcus aureus
Streptococcus pneumoniae
Streptococcus pyogenes
Haemophilus influenzae
Haemophilus parainfluenzae
Moraxella catarrhalis
in vitro but their clinical significance is unknown
in vitro
Staphylococcus epidermidis
Streptococcus agalactiae
Enterococcus Staphylococcus
Citrobacter diversus
Escherichia coli
Klebsiella pneumoniae
Proteus mirabilis
Pseudomonas Enterobacter
(1)
Haemophilus Streptococcus
| MIC (mcg/mL) | Interpretation |
| ≤1 | Susceptible (S) |
| 2 | Intermediate (I) |
| ≥4 | Resistant (R) |

Streptococcus
Streptococcus pneumoniae S. pneumoniae

(2)
Haemophilus Streptococcus d
e


Isolates of Streptococcus pneumoniae should be tested against a 1 mcg oxacillin disk. Isolates with oxacillin zone sizes ≥20 mm are susceptible to penicillin and can be considered susceptible to cefdinir. Streptococci other than S. pneumoniae should be tested with a 10 unit penicillin disk. Isolates with penicillin zone sizes ≥28 mm are susceptible to penicillin and can be considered susceptible to cefdinir.
As with standardized dilution techniques, diffusion methods require the use
of laboratory control microorganisms to control the technical aspects of
laboratory procedures. For the diffusion technique, the 5 mcg cefdinir disk
should provide the following zone diameters in these laboratory quality control
strains:
CEFDINIR INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefdinir capsules and other antibacterial drugs, cefdinir capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Cefdinir capsules are indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Caused by Haemophilus influenzae (including β-lactamase producing strains), Haemophilus parainfluenzae (including β-lactamase producing strains), Streptococcus pneumoniae (penicillin-susceptible strains only), and Moraxella catarrhalis (including β-lactamase producing strains) (see CLINICAL STUDIES).
Caused by Haemophilus influenzae (including β-lactamase producing strains), Haemophilus parainfluenzae (including β-lactamase producing strains), Streptococcus pneumoniae (penicillin-susceptible strains only), and Moraxella catarrhalis (including β-lactamase producing strains).
Caused by Haemophilus influenzae (including β-lactamase producing strains), Streptococcus pneumoniae (penicillin-susceptible strains only), and Moraxella catarrhalis (including β-lactamase producing strains).
NOTE: For information on use in pediatric patients, see Pediatric Use and DOSAGE AND ADMINISTRATION.
Caused by Streptococcus pyogenes (see CLINICAL STUDIES).
NOTE: Cefdinir is effective in the eradication of S. pyogenes from the oropharynx. Cefdinir has not, however, been studied for the prevention of rheumatic fever following S. pyogenes pharyngitis/tonsillitis. Only intramuscular penicillin has been demonstrated to be effective for the prevention of rheumatic fever.
Caused by Staphylococcus aureus (including β-lactamase producing strains) and Streptococcus pyogenes.
Pediatric Patients:
Caused by Haemophilus influenzae (including β-lactamase producing strains), Streptococcus pneumoniae (penicillin-susceptible strains only), and Moraxella catarrhalis (including β-lactamase producing strains).
Caused by Streptococcus pyogenes (see CLINICAL STUDIES).
NOTE: Cefdinir is effective in the eradication of S. pyogenes from the oropharynx. Cefdinir has not, however, been studied for the prevention of rheumatic fever following S. pyogenes pharyngitis/tonsillitis. Only intramuscular penicillin has been demonstrated to be effective for the prevention of rheumatic fever.
Caused by Staphylococcus aureus (including β-lactamase producing strains) and Streptococcus pyogenes.
CEFDINIR CONTRAINDICATIONS
WARNINGS
BEFORE THERAPY WITH CEFDINIR IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFDINIR, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF CEFDINIR IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG β-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFDINIR OCCURS, THE DRUG SHOULD BE DISCONTINUED. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Cefdinir, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
PRECAUTIONS
Prescribing cefdinir capsules in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug resistant bacteria.
As with other broad-spectrum antibiotics, prolonged treatment may result in the possible emergence and overgrowth of resistant organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate alternative therapy should be administered.
Cefdinir, as with other broad-spectrum antimicrobials (antibiotics), should be prescribed with caution in individuals with a history of colitis.
In patients with transient or persistent renal insufficiency (creatinine clearance less than 30 mL/min), the total daily dose of cefdinir should be reduced because high and prolonged plasma concentrations of cefdinir can result following recommended doses (see DOSAGE AND ADMINISTRATION).
Information for Patients:
Patients should be counseled that antibacterial drugs including cefdinir capsules should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefdinir capsules is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefdinir capsules or other antibacterial drugs in the future.
Antacids containing magnesium or aluminum interfere with the absorption of cefdinir. If this type of antacid is required during cefdinir therapy, cefdinir should be taken at least 2 hours before or after the antacid.
Iron supplements, including multivitamins that contain iron, interfere with the absorption of cefdinir. If iron supplements are required during cefdinir therapy, cefdinir should be taken at least 2 hours before or after the supplement.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Interactions:
Concomitant administration of 300 mg cefdinir capsules with 30 mL Maalox® TC suspension reduces the rate (Cmax) and extent (AUC) of absorption by approximately 40%. Time to reach Cmax is also prolonged by 1 hour. There are no significant effects on cefdinir pharmacokinetics if the antacid is administered 2 hours before or 2 hours after cefdinir. If antacids are required during cefdinir capsules therapy, cefdinir capsules should be taken at least 2 hours before or after the antacid.
As with other β-lactam antibiotics, probenecid inhibits the renal excretion of cefdinir, resulting in an approximate doubling in AUC, a 54% increase in peak cefdinir plasma levels, and a 50% prolongation in the apparent elimination t1/2.
Concomitant administration of cefdinir with a therapeutic iron supplement containing 60 mg of elemental iron (as FeSO4) or vitamins supplemented with 10 mg of elemental iron reduced extent of absorption by 80% and 31%, respectively. If iron supplements are required during cefdinir therapy, cefdinir should be taken at least 2 hours before or after the supplement.
The effect of foods highly fortified with elemental iron (primarily iron-fortified breakfast cereals) on cefdinir absorption has not been studied.
There have been reports of reddish stools in patients receiving cefdinir. In many cases, patients were also receiving iron-containing products. The reddish color is due to the formation of a nonabsorbable complex between cefdinir or its breakdown products and iron in the gastrointestinal tract.
Drug/Laboratory Test Interactions
®®®Carcinogenesis, Mutagenesis, Impairment of Fertility:
in vitro in vivo 2Pregnancy:
Cefdinir was not teratogenic in rats at oral doses up to 1000 mg/kg/day (70 times the human dose based on mg/kg/day, 11 times based on mg/m2/day) or in rabbits at oral doses up to 10 mg/kg/day (0.7 times the human dose based on mg/kg/day, 0.23 times based on mg/m2/day). Maternal toxicity (decreased body weight gain) was observed in rabbits at the maximum tolerated dose of 10 mg/kg/day without adverse effects on offspring. Decreased body weight occurred in rat fetuses at ≥100 mg/kg/day, and in rat offspring at ≥32 mg/kg/day. No effects were observed on maternal reproductive parameters or offspring survival, development, behavior, or reproductive function.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery:
Nursing Mothers:
Pediatric Use:
ADVERSE EVENTS
In clinical trials, 5093 adult and adolescent patients (3841 U.S. and 1252 non-U.S.) were treated with the recommended dose of cefdinir capsules (600 mg/day). Most adverse events were mild and self-limiting. No deaths or permanent disabilities were attributed to cefdinir. One hundred forty-seven of 5093 (3%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or definitely associated with cefdinir therapy. The discontinuations were primarily for gastrointestinal disturbances, usually diarrhea or nausea. Nineteen of 5093 (0.4%) patients were discontinued due to rash thought related to cefdinir administration.
In the U.S., the following adverse events were thought by investigators to be
possibly, probably, or definitely related to cefdinir capsules in multiple-dose
clinical trials (N = 3841 cefdinir-treated patients):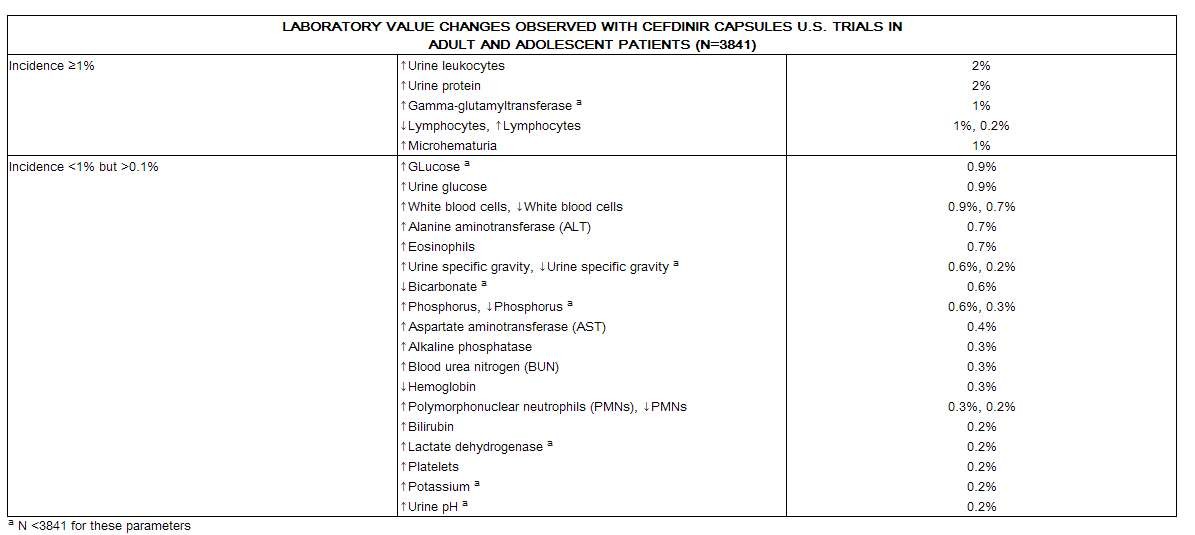
The following laboratory value changes of possible clinical significance,
irrespective of relationship to therapy with cefdinir, were seen during clinical
trials conducted in the U.S.:
The following adverse experiences and altered laboratory tests, regardless of their relationship to cefdinir, have been reported during extensive postmarketing experience, beginning with approval in Japan in 1991: shock, anaphylaxis with rare cases of fatality, facial and laryngeal edema, feeling of suffocation, serum sickness-like reactions, conjunctivitis, stomatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, exfoliative dermatitis, erythema multiforme, erythema nodosum, acute hepatitis, cholestasis, fulminant hepatitis, hepatic failure, jaundice, increased amylase, acute enterocolitis, bloody diarrhea, hemorrhagic colitis, melena, pseudomembranous colitis, pancytopenia, granulocytopenia, leukopenia, thrombocytopenia, idiopathic thrombocytopenic purpura, hemolytic anemia, acute respiratory failure, asthmatic attack, drug-induced pneumonia, eosinophilic pneumonia, idiopathic interstitial pneumonia, fever, acute renal failure, nephropathy, bleeding tendency, coagulation disorder, disseminated intravascular coagulation, upper GI bleed, peptic ulcer, ileus, loss of consciousness, allergic vasculitis, possible cefdinir-diclofenac interaction, cardiac failure, chest pain, myocardial infarction, hypertension, involuntary movements, and rhabdomyolysis.
The following adverse events and altered laboratory tests have been reported for cephalosporin-class antibiotics in general:
Allergic reactions, anaphylaxis, Stevens-Johnson syndrome, erythema multiforme, toxic epidermal necrolysis, renal dysfunction, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anemia, hemolytic anemia, hemorrhage, false-positive test for urinary glucose, neutropenia, pancytopenia, and agranulocytosis. Pseudomembranous colitis symptoms may begin during or after antibiotic treatment (see WARNINGS).
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced (see DOSAGE AND ADMINISTRATION and OVERDOSAGE). If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
CEFDINIR DOSAGE AND ADMINISTRATION
(see INDICATIONS AND USAGE for Indicated Pathogens)
The recommended dosage and duration of treatment for infections in adults and
adolescents are described in the following chart; the total daily dose for all
infections is 600 mg. Once-daily dosing for 10 days is as effective as BID
dosing. Once-daily dosing has not been studied in pneumonia or skin infections;
therefore, cefdinir capsules should be administered twice daily in these
infections. Cefdinir capsules may be taken without regard to meals.
For adult patients with creatinine clearance less than 30 mL/min, the dose of cefdinir should be 300 mg given once daily.
Creatinine clearance is difficult to measure in outpatients. However, the following formula may be used to estimate creatinine clearance (CLcr) in adult patients. For estimates to be valid, serum creatinine levels should reflect steady-state levels of renal function.
(weight) (140 – age)
Males: CLcr = ——————————
(72) (serum creatinine)
Females: CLcr = 0.85 x above value
where creatinine clearance is in mL/min, age is in years, weight is in kilograms, and serum creatinine is in mg/dL.(3)
The following formula may be used to estimate creatinine clearance in pediatric patients:
body length or height
CLcr = K x ——————————
serum creatinine
where K = 0.55 for pediatric patients older than 1 year(4) and 0.45 for infants (up to 1 year)(5).
In the above equation, creatinine clearance is in mL/min/1.73 m2, body length or height is in centimeters, and serum creatinine is in mg/dL.
For pediatric patients with a creatinine clearance of less than 30 mL/min/1.73 m2, the dose of cefdinir should be 7 mg/kg (up to 300 mg) given once daily.
Hemodialysis removes cefdinir from the body. In patients maintained on chronic hemodialysis, the recommended initial dosage regimen is a 300 mg or 7 mg/kg dose every other day. At the conclusion of each hemodialysis session, 300 mg (or 7 mg/kg) should be given. Subsequent doses (300 mg or 7 mg/kg) are then administered every other day.
HOW SUPPLIED
Cefdinir capsules USP, 300 mg, size ‘0’ capsules having blue cap imprinted twice with "LUPIN" (in black ink) and purple body imprinted twice with "CEFDINIR" (in white ink) containing off white to creamish granular slug, are available as follows:
20 Capsules/Bottle NDC 67296-0469-1
Store the capsules at 20°-25°C (68°-77°F); [see USP Controlled Room Temperature].
CLINICAL STUDIES
|
|
Cefdinir
BID
|
Cefaclor
TID
|
Outcome
|
| Clinical Cure Rates Eradication Rates |
150/187 (80%) |
147/186 (79%) |
Cefdinir equivalent to control |
| Overall |
177/195 (91%) |
184/200 (92%) |
Cefdinir equivalent to control |
|
S
.
pneumoniae
|
31/31 (100%) |
35/35 (100%) |
|
|
H
.
influenzae
|
55/65 (85%) |
60/72 (83%) |
|
|
M
.
catarrhalis
|
10/10 (100%) |
11/11 (100%) |
|
|
H
.
parainfluenzae
|
81/89 (91%) |
78/82 (95%) |
|
|
Cefdinir
BID
|
Amoxicillin
/
Clavulanate TID |
Outcome
|
| Clinical Cure Rates Eradication Rates |
83/104 (80%) |
86/97 (89%) |
Cefdinir not equivalent to control |
| Overall |
85/96 (89%) |
84/90 (93%) |
Cefdinir equivalent to control |
|
S
.
pneumoniae
|
42/44 (95%) |
43/44 (98%) |
|
|
H
.
influenzae
|
26/35 (74%) |
21/26 (81%) |
|
|
M
.
catarrhalis
|
6/6 (100%) |
8/8 (100%) |
|
|
H
.
parainfluenzae
|
11/11 (100%) |
12/12 (100%) |
|
In four controlled studies conducted in the U.S., cefdinir
was compared with 10 days of penicillin in adult, adolescent, and pediatric
patients. Two studies (one in adults and adolescents, the other in pediatric
patients) compared 10 days of cefdinir QD or BID to penicillin 250 mg or 10
mg/kg QID. Using strict evaluability and microbiologic/clinical response
criteria 5 to 10 days posttherapy, the following clinical cure rates,
microbiologic eradication rates, and statistical outcomes were obtained:
|
Study
|
Efficacy
Parameter
|
Cefdinir
QD
|
Cefdinir
BID
|
Penicillin
QID
|
Outcome
|
| Adults/ Adolescents |
Eradication of S
.
pyogenes
|
192/210 (91%) |
199/217 (92%) |
181/217 (83%) |
Cefdinir superior to control |
|
|
Clinical Cure Rates |
199/210 (95%) |
209/217 (96%) |
193/217 (89%) |
Cefdinir superior to control |
| Pediatric Patients |
Eradication of S
.
pyogenes
|
215/228 (94%) |
214/227 (94%) |
159/227 (70%) |
Cefdinir superior to control |
|
|
Clinical Cure Rates |
222/228 (97%) |
218/227 (96%) |
196/227 (86%) |
Cefdinir superior to control |
Two studies (one in adults and adolescents, the other in pediatric patients) compared 5 days of cefdinir BID to 10 days of penicillin 250 mg or 10 mg/kg QID. Using strict evaluability and microbiologic/ clinical response criteria 4 to 10 days posttherapy, the following clinical cure rates, microbiologic eradication rates, and statistical outcomes were obtained:
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 4th ed. Approved Standard, NCCLS Document M7-A4, Vol 17(2). NCCLS, Villanova, PA, Jan 1997.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests, 6th ed. Approved Standard, NCCLS Document M2-A6, Vol 17(1). NCCLS, Villanova, PA, Jan 1997.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron, 1976;16:31-41.
- Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976;58:259-63.
- Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatrics 1984;104:849-54.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Mandideep 462 046
INDIA
Maalox® is a registered trademark of Rhone-Poulenc Rorer.
Clinistix® and Clinitest® are registered trademarks of Miles Diagnostics.
Tes-tape® is a registered trademark of Lilly.
Revised 15th December 2009 ID#:218628

CefdinirCefdinir CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||