CEFPODOXIME PROXETIL
PD-Rx Pharmaceuticals, Inc.
PD-Rx Pharmaceuticals, Inc.
Cefpodoxime Proxetil Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- CEFPODOXIME PROXETIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- CEFPODOXIME PROXETIL INDICATIONS AND USAGE
- CEFPODOXIME PROXETIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CEFPODOXIME PROXETIL ADVERSE REACTIONS
- OVERDOSAGE
- CEFPODOXIME PROXETIL DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- CLINICAL TRIALS
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 200 mg (100 Tablet Bottle)
FULL PRESCRIBING INFORMATION
For Oral Use Only
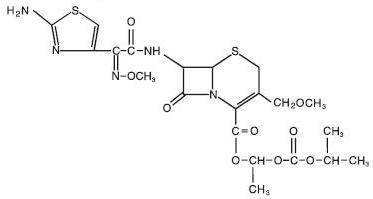
CEFPODOXIME PROXETIL DESCRIPTION
2127592
CLINICAL PHARMACOLOGY
Absorption and Excretion
in vivo
Effects of Food
max
Pharmacokinetics of Cefpodoxime Proxetil Film-coated Tablets
max max1/2max
| Dose (cefpodoxime equivalents) |
Time after oral ingestion | ||||||
|---|---|---|---|---|---|---|---|
| 1hr | 2hr | 3hr | 4hr | 6hr | 8hr | 12hr | |
| 100 mg |
0.98 |
1.4 |
1.3 |
1 |
0.59 |
0.29 |
0.08 |
| 200 mg |
1.5 |
2.2 |
2.2 |
1.8 |
1.2 |
0.62 |
0.18 |
| 400 mg |
2.2 |
3.7 |
3.8 |
3.3 |
2.3 |
1.3 |
0.38 |
Pharmacokinetics of Cefpodoxime Proxetil Suspension
| Dose (cefpodoxime equivalents) |
Time after oral ingestion | ||||||
|---|---|---|---|---|---|---|---|
| 1hr | 2hr | 3hr | 4hr | 6hr | 8hr | 12hr | |
|
1 Dose did not exceed 200 mg. |
|||||||
| 5 mg/kg1
|
1.4 |
2.1 |
2.1 |
1.7 |
0.90 |
0.40 |
0.090 |
Distribution
Skin Blister
Tonsil Tissue
90 S. pyogenes
Lung Tissue
90
CSF
Effects of Decreased Renal Function
Elimination of cefpodoxime is reduced in patients with moderate to severe renal impairment (<50 mL/min creatinine clearance). (See PRECAUTIONS and DOSAGE AND ADMINISTRATION.) In subjects with mild impairment of renal function (50 to 80 mL/min creatinine clearance), the average plasma half-life of cefpodoxime was 3.5 hours. In subjects with moderate (30 to 49 mL/min creatinine clearance) or severe renal impairment (5 to 29 mL/min creatinine clearance), the half-life increased to 5.9 and 9.8 hours, respectively. Approximately 23% of the administered dose was cleared from the body during a standard 3-hour hemodialysis procedure.
Effect of Hepatic Impairment (cirrhosis)
1/2
Pharmacokinetics in Elderly Subjects
PRECAUTIONS.maxmax
Microbiology
in vitro INDICATIONS AND USAGE
Aerobic Gram-positive microorganisms
Staphylococcus aureu
Staphylococcus saprophyticus
Streptococcus pneumoniae
Streptococcus pyogenes
Aerobic Gram-negative microorganisms
Escherichia coli
Klebsiella pneumoniae
Proteus mirabilis
Haemophilus influenzae
Moraxella (Branhamella) catarrhalis
Neisseria gonorrhoeae
in vitroin vitro
Aerobic Gram-positive microorganisms
Streptococcus agalactiae
Streptococcus spp.
Aerobic Gram-negative microorganisms
Citrobacter diversus
Klebsiella oxytoca
Proteus vulgaris
Providencia rettgeri
Haemophilus parainfluenzae
Pseudomonas Enterobacter
Anaerobic Gram-positive microorganisms
Peptostreptococcus magnus
SUSCEPTIBILITY TESTING
Dilution Techniques: 1,2
For Susceptibility Testing of Enterobacteriaceae and Staphylococcus spp.
MIC (mcg/mL)Interpretation
For Susceptibility Testing of Haemophilus spp. a
MIC (mcg/mL)Interpretationb
a Haemophilus spp.2
b
For Susceptibility Testing of Neisseria gonorrhoeae. c
MIC (mcg/mL)Interpretationd
c N. gonorroheae Neisseria gonorrhoeae2
d
For Susceptibility Testing of Streptococcus pneumoniae.
MIC (mcg/mL)Interpretatione
eS. pneumoniae2
For Susceptibility Testing of Streptococcus spp. other than Streptococcus pneumoniae. f
fStreptococcus spp2
Quality Control
Microorganism (ATCC®#)MIC Range (mcg/mL)
Escherichia coli
Staphylococcus aureus
Haemophilus influenzae g
Neisseria gonorrhoeae h
Streptococcus pneumoniaeji
g
h
i
j Streptococcus pneumoniaeStreptococcus spp
Diffusion Techniques : 3
For Susceptibility Testing of Enterobacteriaceae and Staphylococcus spp.
Zone Diameter (mm)Interpretation
For Susceptibility Testing of Haemophilus spp. k
Zone Diameter (mm)Interpretationl
k Haemophilus spp.22
l
For Susceptibility Testing of Neisseria gonorrhoeae. m
Zone Diameter (mm)Interpretationn
m N. gonorrhoeae 22
n
For Susceptibility Testing of Streptococcus pneumoniae. o
oS. pneumoniae22
For Susceptibility Testing of Streptococcus spp. other than Streptococcus pneumoniae.p
pStreptococcus spp.22
Quality Control
Microorganism (ATCC®#)Zone Diameter Range (mm)
Escherichia coli
Staphylococcus aureus
Haemophilus influenzaeq
Neisseria gonorrhoeaer
Streptococcus pneumoniaets
q 2
r 2
s 2
t S. pneumoniae Streptococcus spp.
®
CEFPODOXIME PROXETIL INDICATIONS AND USAGE
Recommended dosages, durations of therapy, and applicable patient populations vary among these infections. Please see DOSAGE AND ADMINISTRATION for specific recommendations. Acute otitis mediaStreptococcus pneumoniaeStreptococcus pyogenesHaemophilus influenzaeMoraxella (Branhamella)catarrhalis
Pharyngitis and/or tonsillitis Streptococcus pyogenes
NOTE:
Community-acquired pneumoniaS. pneumoniae H. Influenzae
Acute bacterial exacerbation of chronic bronchitisS. pneumoniaeH. influenzae M. catarrhalisH. influenzae
Acute, uncomplicated urethral and cervical gonorrheaNeisseria gonorrhoeae
Acute, uncomplicated ano-rectal infections in womenNeisseria gonorrhoeae
NOTE: N. gonorrhoeaeN. gonorrhoeae
Uncomplicated skin and skin structure infectionsStaphylococcus aureusStreptococcus pyogenes
NOTE: DOSAGE AND ADMINISTRATION
Acute maxillary sinusitisHaemophilus influenzae Streptococcus pneumoniaeMoraxella catarrhalis
Uncomplicated urinary tract infections (cystitis)Escherichia coli, Klebsiella pneumoniae, Proteus mirabilisStaphylococcus saprophyticus
NOTE: CLINICAL STUDIES
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefpodoxime proxetil and other antibacterial drugs, cefpodoxime proxetil should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CEFPODOXIME PROXETIL CONTRAINDICATIONS
WARNINGS
BEFORE THERAPY WITH CEFPODOXIME PROXETIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPODOXIME, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF CEFPODOXIME IS TO BE ADMINISTERED TO PENICILLIN SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFPODOXIME PROXETIL OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINE, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile C. difficile
C. difficile C. difficile
C. difficileC. difficile
C. difficile C. difficileC. difficile
PRECAUTIONS
General
DOSAGE AND ADMINISTRATION
Information for Patients
Drug Interactions
Antacids:2max
Probenecid:
Nephrotoxic drugs:
Drug/Laboratory Test Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vivo2
Pregnancy
Teratogenic effects
22
Labor and Delivery
Nursing Mothers
Pediatric Use
Geriatric Use
CEFPODOXIME PROXETIL ADVERSE REACTIONS
Clinical Trials
Film-coated Tablets (Multiple dose)
multiple doses
Incidence Greater Than 1%
C. difficile WARNINGS
Incidence Less Than 1%: By body system in decreasing order:
Clinical Studies
Adverse events thought possibly or probably related to cefpodoxime proxetil that occurred in less than 1% of patients (N=4696)
Body
Cardiovascular
Digestive
Hemic and Lymphatic
Metabolic and Nutritional
Musculo-skeletal
Nervous
Respiratory
Skin
Special Senses
Urogenital
Granules for Oral Suspension (Multiple dose)
Incidence Greater Than 1%:
Incidence Less Than 1%:
Body:
Digestive:
Hemic & Lymphatic:
Metabolic & Nutritional:
Musculo-Skeletal:
Nervous:
Respiratory:
Skin:
Special Senses:
Film-coated Tablets (Single dose)
single dose
Incidence Greater Than 1%:
Incidence Less Than 1%:
Central Nervous System:
Dermatologic:
Genital:
Gastrointestinal:
Psychiatric:
Laboratory Changes
Hepatic:
Hematologic:
Serum Chemistry:
Renal:
Post-marketing Experience
Cephalosporin Class Labeling
Adverse Reactions and Abnormal Laboratory Tests:
DOSAGE AND ADMINISTRATION OVERDOSAGE
OVERDOSAGE
CEFPODOXIME PROXETIL DOSAGE AND ADMINISTRATION
(See INDICATIONS AND USAGE for indicated pathogens.)
FILM-COATED TABLETS
CLINICAL PHARMACOLOGY
| Type of Infection | Total Daily Dose |
Dose Frequency | Duration |
|---|---|---|---|
| Pharyngitis and/or tonsillitis |
200 mg |
100 mg Q 12 hours |
5 to 10 days |
| Acute community-acquired pneumonia |
400 mg |
200 mg Q 12 hours |
14 days |
| Acute bacterial exacerbations of chronic bronchitis |
400 mg |
200 mg Q 12 hours |
10 days |
| Uncomplicated gonorrhea (men and women) and rectal gonococcal infections (women) |
200 mg |
single dose |
|
| Skin and skin structure |
800 mg |
400 mg Q 12 hours |
7 to 14 days |
| Acute maxillary sinusitis |
400 mg |
200 mg Q 12 hours |
10 days |
| Uncomplicated urinary tract infection |
200 mg |
100 mg Q 12 hours |
7 days |
GRANULES FOR ORAL SUSPENSION
| Type of Infection | Total Daily Dose | Dose Frequency | Duration |
|---|---|---|---|
| Pharyngitis and/or tonsillitis |
200 mg |
100 mg Q 12 hours |
5 to 10 days |
| Acute community-acquired pneumonia |
400 mg |
200 mg Q 12 hours |
14 days |
| Uncomplicated gonorrhea (men and women) and rectal gonococcal infections (women) |
200 mg |
single dose |
|
| Skin and skin structure |
800 mg |
400 mg Q 12 hours |
7 to 14 days |
| Acute maxillary sinusitis |
400 mg |
200 mg Q 12 hours |
10 days |
| Uncomplicated urinary tract infection |
200 mg |
100 mg Q 12 hours |
7 days |
| Type of Infection | Total Daily Dose | Dose Frequency | Duration |
|---|---|---|---|
| Acute otitis media |
10 mg/kg/day (Max 400 mg/day) |
5 mg/kg Q 12 h (Max 200 mg/dose) |
5 days |
| Pharyngitis and/or tonsillitis |
10 mg/kg/day (Max 200 mg/day) |
5 mg/kg/dose Q 12 h (Max 100 mg/dose) |
5 to 10 days |
| Acute maxillary sinusitis |
10 mg/kg/day (Max 400 mg/day) |
5 mg/kg Q 12 hours (Max 200 mg/dose) |
10 days |
Patients with Renal Dysfunction
Weight (kg) x (140 - age)
Patients with Cirrhosis
HOW SUPPLIED
Cefpodoxime Proxetil Tablets, USP 100 mg
Cefpodoxime Proxetil Tablets, USP 200 mg
Store at
REFERENCES
- NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically –fourth edition; Approved standard. NCCLS document M7-A4 (ISBN 1-56238-309-4). NCCLS, 940 West Valley Rd., Suite 1400, Wayne, PA 19087-1898, 1997.
- NCCLS. Performance standards for antimicrobial susceptibility testing; Eighth informational supplement. NCCLS document M100-S8 (ISBN 1-56238-337-x). NCCLS, 940 West Valley Rd., Suite 1400, Wayne, PA 19087-1898, 1998.
- NCCLS. Performance standards for antimicrobial disk susceptibility tests - sixth edition; Approved standard. NCCLS document M2-A6 (ISBN 1-56238-306-6). NCCLS, 940 West Valley Rd., Suite 1400, Wayne, PA 19087-1898, 1997.
CLINICAL TRIALS
Cystitis
| Pathogen | Cefpodoxime | Comparator |
|---|---|---|
|
E. coli
|
200/243 (82%) |
99/123 (80%) |
| Other pathogens |
34/42 (81%) |
23/28 (82%) |
|
K. pneumoniae
P. mirabilis S. saprophyticus |
|
|
| TOTAL
|
234/285 (82%) |
122/151 (81%) |
Acute Otitis Media Studies
| Cefpodoxime Proxetil | Cefixime | |
|---|---|---|
|
Pathogen
|
5 mg/kg Q 12 h x 5 d
|
|
|
S. pneumoniae
|
88/122 (72%) |
72/124 (58%) |
|
H. influenzae
|
50/76 (66%) |
61/81 (75%) |
|
M. catarrhalis
|
22/39 (56%) |
23/41 (56%) |
|
S. pyogenes
|
20/25 (80%) |
13/23 (57%) |
| Clinical success rate
|
171/254 (67%) |
165/258 (64%) |
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 200 mg (100 Tablet Bottle)
Cefpodoxime Proxetil
Tablets, USP 200 mg*
Rx only 100 Tablets
AUROBINDO

CEFPODOXIME PROXETILCEFPODOXIME PROXETIL TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||