Cefprozil
Northstar Rx LLC
Aurobindo Pharma Limited
FULL PRESCRIBING INFORMATION: CONTENTS*
- CEFPROZIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- CEFPROZIL INDICATIONS AND USAGE
- CEFPROZIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CEFPROZIL ADVERSE REACTIONS
- OVERDOSAGE
- CEFPROZIL DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- REFERENCES
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 125 mg/5 mL (50 mL Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg/5 mL (50 mL Bottle)
FULL PRESCRIBING INFORMATION
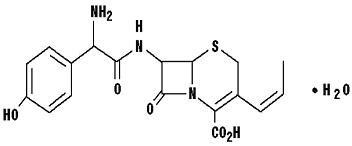
CEFPROZIL DESCRIPTION
6R, 7RRp
1819352
CLINICAL PHARMACOLOGY
| *Data represent mean values of 12 healthy volunteers. |
||||
| Dosage (mg) |
Mean Plasma Cefprozil Concentrations (mcg/mL)* |
8-hour Urinary Excretion (%) |
||
| Peak appx. 1.5 h |
4 h |
8 h |
||
| 250 mg |
6.1 |
1.7 |
0.2 |
60% |
| 500 mg |
10.5 |
3.2 |
0.4 |
62% |
| 1000 mg |
18.3 |
8.4 |
1 |
54% |
maxmax
PRECAUTIONS DOSAGE AND ADMINISTRATION
|
an=11; bn=5; cn=9; dn=11. |
||||||
| |
Mean (SD) Plasma Cefprozil Concentrations (mcg/mL) |
|||||
| Population |
Dose |
1 h |
2 h |
4 h |
6 h |
T1/2 (h) |
| children (n=18) |
7.5 mg/kg |
4.7 (1.57) |
3.99 (1.24) |
0.91 (0.3) |
0.23a
(0.13) |
0.94 (0.32) |
| adults (n=12) |
250 mg |
4.82 (2.13) |
4.92 (1.13) |
1.7b
(0.53) |
0.53 (0.17) |
1.28 (0.34) |
| children (n=19) |
15 mg/kg |
10.86 (2.55) |
8.47 (2.03) |
2.75 (1.07) |
0.61c
(0.27) |
1.24 (0.43) |
| adults (n=12) |
500 mg |
8.39 (1.95) |
9.42 (0.98) |
3.18d
(0.76) |
1d
(0.24) |
1.29 (0.14) |
| children (n=10) |
30 mg/kg |
16.69 (4.26) |
17.61 (6.39) |
8.66 (2.7) |
– |
2.06 (0.21) |
| adults (n=12) |
1000 mg |
11.99 (4.67) |
16.95 (4.07) |
8.36 (4.13) |
2.79 (1.77) |
1.27 (0.12) |
Microbiology
in vitroin vitro INDICATIONS AND USAGE
| Aerobic gram-positive microorganisms: | Aerobic gram-negative microorganisms: |
|---|---|
|
Staphylococcus aureus
(including β-lactamase-producing strains) |
Haemophilus influenzae
(including β-lactamase-producing strains) |
|
NOTE: Cefprozil is inactive against methicillin-resistant staphylococci. |
Moraxella (Branhamella) catarrhalis
(including β-lactamase-producing strains) |
|
Streptococcus pneumoniae
|
|
|
Streptococcus pyogenes
|
|
in vitroin vitro
Aerobic gram-positive microorganisms:
Enterococcus durans Staphylococcus warneri
Enterococcus faecalis Streptococcus agalactiae
Listeria monocytogenes
Staphylococcus epidermidis
Staphylococcus saprophyticus
NOTE:Enterococcus faecium
Aerobic gram-negative microorganisms:
Citrobacter diversus Proteus mirabilis
Escherichia coli Salmonella
Klebsiella pneumoniae Shigella .
Neisseria gonorrhoeae Vibrio
NOTE: AcinetobacterEnterobacterMorganella morganiiProteus vulgarisProvidenciaPseudomonasSerratia
Anaerobic microorganisms:
Prevotella (Bacteroides) melaninogenicus Fusobacterium
Clostridium difficile Peptostreptococcus
Clostridium perfringens Propionibacterium acnes
NOTE: Bacteroides fragilis
Susceptibility Tests
Dilution Techniques: 1,2
| MIC (mcg/mL) | Interpretation |
|---|---|
| ≤8 |
Susceptible (S) |
| 16 |
Intermediate (I) |
| ≥32 |
Resistant (R) |
| Microorganism | MIC (mcg/mL) |
|---|---|
|
Enterococcus faecalis ATCC 29212 |
4–16 |
|
Escherichia coli ATCC 25922 |
1–4 |
|
Haemophilus influenzae ATCC 49766 |
1–4 |
|
Staphylococcus aureus ATCC 29213 |
0.25–1 |
|
Streptococcus pneumoniae ATCC 49619 |
0.25–1 |
Diffusion Techniques:3
| Zone diameter (mm) | Interpretation |
|---|---|
| ≥18 |
Susceptible (S) |
| 15-17 |
Intermediate (I) |
| ≤14 |
Resistant (R) |
| Microorganism | Zone diameter (mm) |
|---|---|
|
Escherichia coli ATCC 25922 |
21–27 |
|
Haemophilus influenzae ATCC 49766 |
20–27 |
|
Staphylococcus aureus ATCC 25923 |
27–33 |
|
Streptococcus pneumoniae ATCC 49619 |
25–32 |
CEFPROZIL INDICATIONS AND USAGE
Pharyngitis/tonsillitis Streptococcus pyogenes
Streptococcus pyogenes
Otitis Media Streptococcus pneumoniaeHaemophilus influenzaeMoraxella (Branhamella) catarrhalis CLINICAL STUDIES
Acute Sinusitis Streptococcus pneumoniaeHaemophilus influenzae Moraxella (Branhamella) catarrhalis
Secondary Bacterial Infection of Acute Bronchitis and Acute Bacterial Exacerbation of Chronic Bronchitis Streptococcus pneumoniaeHaemophilus influenzae Moraxella (Branhamella) catarrhalis
Uncomplicated Skin and Skin-Structure InfectionsStaphylococcus aureus Streptococcus pyogenes
CEFPROZIL CONTRAINDICATIONS
WARNINGS
BEFORE THERAPY WITH CEFPROZIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPROZIL, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-SENSITIVITY AMONG β-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFPROZIL OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile C. difficile
C. difficile C. difficile
C. difficile C. difficile
PRECAUTIONS
General
DOSAGE AND ADMINISTRATION
Information for Patients
Drug Interactions
Drug/Laboratory Test Interactions
®®
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vivo
Salmonella E. coli in vitro2
2
Pregnancy
Teratogenic effects
Pregnancy Category B
2
Labor and Delivery
Nursing Mothers
Pediatric Use
(See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION.)
CLINICAL STUDIES
Geriatric Use
CLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
CEFPROZIL ADVERSE REACTIONS
Gastrointestinal:
Hepatobiliary:
Hypersensitivity:
CNS:
Hematopoietic:
Renal:
Other:
Cephalosporin class paragraph
DOSAGE AND ADMINISTRATION OVERDOSAGE
OVERDOSAGE
CEFPROZIL DOSAGE AND ADMINISTRATION
| Population/Infection | Dosage (mg) |
Duration (days) |
|---|---|---|
|
a In the treatment of infections due to Streptococcus pyogenes, cefprozil should be administered for at least 10 days. b Not to exceed recommended adult doses. |
||
| ADULTS (13 years and older) |
|
|
| UPPER RESPIRATORY TRACT |
|
|
| Pharyngitis/Tonsillitis |
500 q24h |
10a
|
| Acute Sinusitis (For moderate to severe infections, the higher dose should be used) |
250 q12h or 500 q12h |
10 |
| LOWER RESPIRATORY TRACT |
|
|
| Secondary Bacterial Infection of Acute Bronchitis and Acute Bacterial Exacerbation of Chronic Bronchitis |
500 q12h |
10 |
| SKIN AND SKIN STRUCTURE |
|
|
| Uncomplicated Skin and Skin Structure Infections |
250 q12h or 500 q24h or 500 q12h |
10 |
| CHILDREN (2 years to 12 years) |
|
|
| UPPER RESPIRATORY TRACTb
|
|
|
| Pharyngitis/Tonsillitis |
7.5 mg/kg q12h |
10a
|
| SKIN AND SKIN STRUCTUREb
|
|
|
| Uncomplicated Skin and Skin Structure Infections |
20 mg/kg q24h |
10 |
| INFANTS & CHILDREN (6 months to 12 years) |
|
|
| UPPER RESPIRATORY TRACTb
|
|
|
| Otitis Media (See INDICATIONS AND USAGE and CLINICAL STUDIES ) |
15 mg/kg q12h |
10 |
| Acute Sinusitis (For moderate to severe infections, the higher dose should be used) |
7.5 mg/kg q12h or 15 mg/kg q12h |
10 |
Renal Impairment
| Creatinine Clearance (mL/min) |
Dosage (mg) |
Dosing Interval |
|---|---|---|
| * Cefprozil is in part removed by hemodialysis; therefore, cefprozil should be administered after the completion of hemodialysis. |
||
| 30–120 0–29* |
standard 50% of standard |
standard standard |
Hepatic Impairment
HOW SUPPLIED
Cefprozil for Oral Suspension, USP 125 mg/5 mL:
Cefprozil for Oral Suspension, USP 250 mg/5 mL: Each 5 mL of reconstituted suspension contains cefprozil USP equivalent to anhydrous cefprozil 250 mg.
Reconstitution Directions for Oral Suspension
|
Bottle Size |
Final Concentration 125 mg/5 mL |
Final Concentration 250 mg/5 mL |
|---|---|---|
| 50 mL |
35 mL |
35 mL |
| 75 mL |
52 mL |
52 mL |
| 100 mL |
70 mL |
70 mL |
Store dry powder at
CLINICAL STUDIES
Study One:
acute otitis media
| EFFICACY: | ||
|---|---|---|
| Pathogen | % of Cases with Pathogen (n=155) |
Outcome |
|
S. pneumoniae
|
48.4% |
cefprozil success rate 5% better than control |
|
H. influenzae
|
35.5% |
cefprozil success rate 17% less than control |
|
M. catarrhalis
|
13.5% |
cefprozil success rate 12% less than control |
|
S. pyogenes
|
2.6% |
cefprozil equivalent to control |
| Overall
|
100% |
cefprozil success rate 5% less than control |
SAFETY:
| Age Group | Cefprozil | Control |
|---|---|---|
| * The majority of these involved the diaper area in young children. |
||
| 6 months to 2 years |
21% |
41% |
| 3 to 12 years |
10% |
19% |
Study Two:
acute otitis media
| EFFICACY: | ||
|---|---|---|
| Pathogen | % of Cases with Pathogen (n=47) |
Outcome |
|
S. pneumoniae
|
51% |
cefprozil equivalent to control |
|
H. influenzae
|
29.8% |
cefprozil equivalent to control |
|
M. catarrhalis
|
6.4% |
cefprozil equivalent to control |
|
S. pyogenes
|
12.8% |
cefprozil equivalent to control |
| Overall |
100% |
cefprozil equivalent to control |
SAFETY:
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically-Third Edition. Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25, NCCLS, Villanova, PA, December 1993.
- National Committee for Clinical Laboratory Standards. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria-Third Edition. Approved Standard NCCLS Document M11-A3, Vol. 13, No. 26, NCCLS, Villanova, PA, December 1993.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests–Fifth Edition. Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA, December 1993.
Clinitest® and Clinistix® are registered trademarks of the Bayer HealthCare LLC.
Manufactured for: Northstar Rx LLC
Memphis, TN 38141
Toll Free: 1-800-206-7821
Manufactured by: Aurobindo Pharma Limited
Chitkul (V)-502 307, A.P; India
M.L.No.: 78/MD/AP/96/F/B/R
Issued: 12/2012
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 125 mg/5 mL (50 mL Bottle)
Rx onlyNDC 16714-396-01
Cefprozil for Oral Suspension, USP
125 mg/5 mL
50 mL
when reconstituted
Northstar Rx LLC

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg/5 mL (50 mL Bottle)
Rx only
NDC 16714-397-01
Cefprozil for Oral Suspension, USP
250 mg/5 mL
50 mL
when reconstituted
Northstar Rx LLC

CefprozilCefprozil POWDER, FOR SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CefprozilCefprozil POWDER, FOR SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!