Ceftiflex
Ceftiofur Sodium Sterile powder
FULL PRESCRIBING INFORMATION: CONTENTS*
- CEFTIFLEX DESCRIPTION
- INDICATIONS
- CEFTIFLEX DOSAGE AND ADMINISTRATION
- CEFTIFLEX CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CEFTIFLEX ADVERSE REACTIONS
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
For intramuscular and subcutaneous injection in cattle only. For intramuscular injection in swine, sheep, goats, and horses. For subcutaneous injection only in dogs, day-old chickens and day-old turkey poults. This product may be used in lactating dairy cattle, sheep, and goats.
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
CEFTIFLEX DESCRIPTION
Ceftiofur Sodium Sterile Powder contains the sodium salt of ceftiofur which is a broad spectrum cephalosporin antibiotic active against gram-positive and gram-negative bacteria including β-lactamase-producing strains. Like other cephalosporins, ceftiofur is bactericidal in vitro, resulting from inhibition of cell wall synthesis.
Each mL of the reconstituted drug contains ceftiofur sodium equivalent to 50 mg ceftiofur. The pH was adjusted with sodium hydroxide and monobasic potassium phosphate.
Chemical structure of Ceftiofur Sodium
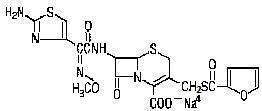
Chemical Name of Ceftiofur Sodium
5-Thia-1-azabicyclo [4.2.0] oct-2-ene-carboxylic acid, 7-[[(2-amino-4-thiazoly)(methoxyimino)-acetyl]amino]-3-[[(2-furanylcarbonyl)thio] methyl]-8-oxo-, monosodium salt, [6R-[6α,7β(Z)]]-
RECONSTITUTION OF THE STERILE POWDER
Ceftiofur Sodium Sterile Powder should be reconstituted as follows:
1 gram vial- Reconstitute with 20 mL Sterile Water for Injection. Each mL of the resulting solution contains ceftiofur sodium equivalent to 50 mg ceftiofur.
4 gram vial- Reconstitute with 80 mL Sterile Water for Injection. Each mL of the resulting solution contains ceftiofur sodium equivalent to 50 mg ceftiofur.
Shake thoroughly prior to use.
INDICATIONS
Cattle
Ceftiofur Sodium Sterile Powder is indicated for treatment of bovine respiratory disease (shipping fever, pneumonia) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni. Ceftiofur Sodium Sterile Powder is also indicated for treatment of acute bovine interdigital necrobacillosis (foot rot, pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus.
Swine
Ceftiofur Sodium Sterile Powder is indicated for treatment/control of swine bacterial respiratory disease (swine bacterial pneumonia) associated with Actinobacillus (Haemophilus) pleuropneumoniae, Pasteurella multocida, Salmonella choleraesuis and Streptococcus suis.
Sheep
Ceftiofur Sodium Sterile Powder is indicated for treatment of sheep respiratory disease (sheep pneumonia) associated with Mannheimia haemolytica and Pasteurella multocida.
Goat
Ceftiofur Sodium Sterile Powder is indicated for treatment of caprine respiratory disease (goat pneumonia) associated with Mannheimia haemolytica and Pasteurella multocida.
Horses
Ceftiofur Sodium Sterile Powder is indicated for treatment of respiratory infections in horses associated with Streptococcus zooepidemicus.
Dogs
Ceftiofur Sodium Sterile Powder is indicated for the treatment of canine urinary tract infections associated with Escherichia coli and Proteus mirabilis.
Day-Old Chicks
Ceftiofur Sodium Sterile Powder is indicated for the control of early mortality, associated with E. coli organisms susceptible to ceftiofur, in day-old chicks.
Day-Old Turkey Poults
Ceftiofur Sodium Sterile Powder is indicated for the control of early mortality, associated with E. coli organisms susceptible to ceftiofur, in day-old turkey poults.
CEFTIFLEX DOSAGE AND ADMINISTRATION
Cattle
Administer to cattle by intramuscular or subcutaneous injection at the dosage of 0.5 to 1.0 mg ceftiofur per pound (1.1 to 2.2 mg/kg) of body weight (1-2 mL reconstituted sterile solution per 100 lbs body weight). Treatment should be repeated at 24-hour intervals for a total of three consecutive days. Additional treatments may be given on days four and five for animals which do not show a satisfactory response (not recovered) after the initial three treatments. Selection of dosage (0.5 to 1.0 mg/lb) should be based on the practitioner's judgment of severity of disease (i.e., for respiratory disease, extent of elevated body temperature, depressed physical appearance, increased respiratory rate, coughing and /or loss of appetite; and for foot rot, extent of swelling, lesion and severity of lameness).
Swine
Administer to swine by intramuscular injection at the dosage of 1.36 to 2.27 mg ceftiofur per pound (3.0 to 5.0 mg/kg) of body weight (1 mL of reconstituted sterile solution per 22 to 37 lbs body weight). Treatment should be repeated at 24-hour intervals for a total of three consecutive days.
Sheep
Administer to sheep by intramuscular injection at the dosage of 0.5 to 1.0 mg ceftiofur per pound (1.1 to 2.2 mg/kg) of body weight (1-2 mL reconstituted sterile solution per 100 lbs body weight). Treatment should be repeated at 24-hour intervals for a total of three consecutive days. Additional treatments may be given on days four and five for animals which do not show a satisfactory response (not recovered) after the initial three treatments. Selection of dosage (0.5 to 1.0 mg/lb) should be based on the practitioner's judgment of severity of disease (i.e., extent of elevated body temperature, depressed physical appearance, increased respiratory rate, coughing and/or loss of appetite).
Goats
Administer to goats by intramuscular injection at the dosage of 0.5 to 1.0 mg ceftiofur per pound (1.1 to 2.2 mg/kg) of body weight (1-2 mL reconstituted sterile solution per 100 lbs body weight). Treatment should be repeated at 24-hour intervals for a total of three consecutive days. Additional treatments may be given on days four and five for animals which do not show a satisfactory response (not recovered) after the initial three treatments. Selection of dosage (0.5 to 1.0 mg/lb) should be based on the practitioner's judgment of severity of disease (i.e., extent of elevated body temperature, depressed physical appearance, increased respiratory rate, coughing and/or loss of appetite). Pharmacokinetic data indicate that elimination of the drug is more rapid in lactating does. For lactating does, the high end of the dose range is recommended.
Horses
Administer to horses by intramuscular injection at the dosage of 1.0 to 2.0 mg ceftiofur per pound (2.2 to 4.4 mg/kg) of body weight (2-4 mL reconstituted sterile solution per 100 lbs body weight). A maximum of 10 mL may be administered per injection site. Treatment should be repeated at 24-hour intervals, continued for 48 hours after clinical signs have disappeared and should not exceed 10 days.
Dogs
Administer to dogs by subcutaneous injection at the dosage of 1.0 mg ceftiofur per pound (2.2 mg/kg) of body weight (0.1 mL reconstituted sterile solution per 5 lbs body weight). Treatment should be repeated at 24-hour intervals for 5-14 days.
Reconstituted Ceftiofur Sodium Sterile Powder is to be administered to dogs by subcutaneous injection. No vial closure should be entered more than 20 times. Therefore, only the 1 gram vial is approved for use in dogs.
Day-Old Chicks
Administer by subcutaneous injection in the neck region of day-old chicks at the dosage of 0.08 to 0.20 mg ceftiofur/chick. One mL of the 50 mg/mL reconstituted solution will treat approximately 250 to 625 day-old chicks.
Reconstituted Ceftiofur Sodium Sterile Powder is to be administered by subcutaneous injection only. A sterile 26 gauge needle and syringe or properly cleaned automatic injection machine should be used.
Day-Old Turkey Poults
Administer by subcutaneous injection in the neck region of day-old turkey poults at the dosage of 0.17 to 0.5 mg ceftiofur/poult. One mL of the 50 mg/mL reconstituted solution will treat approximately 100 to 294 day-old turkey poults.
Reconstituted Ceftiofur Sodium Sterile Powder is to be administered by subcutaneous injection only.
CEFTIFLEX CONTRAINDICATIONS
As with all drugs, the use of Ceftiofur Sodium Sterile Powder is contraindicated in animals previously found to be hypersensitive to the drug.
WARNINGS
NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN.
Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth, and clothing.
Persons with a known hypersensitivity to penicillin or cephalosporins should avoid exposure to this product.
In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g., skin rash, hives, difficult breathing), seek medical attention.
The material safety data sheet contains more detailed occupational safety information. To obtain a material safety data sheet (MSDS) please call 1-909-392-8900. To report any adverse event please call 1-909-392-8900

PRECAUTIONS
The effects of ceftiofur on the reproductive performance, pregnancy, and lactation of cattle, swine, sheep, and goats have not been determined.
Cattle
Following subcutaneous administration of ceftiofur sodium in the neck, small areas of discoloration at the site may persist beyond five days, potentially resulting in trim loss of edible tissues at slaughter.
As with any parenteral injection, localized post-injection bacterial infections may result in abscess formation. Attention to hygienic procedures can minimize their occurrence.
Swine
The safety of ceftiofur has not been determined for swine intended for breeding.
Horses
The safety of ceftiofur has not been determined for horses intended for breeding. The administration of antimicrobials to horses under conditions of stress may be associated with acute diarrhea that could be fatal. If acute diarrhea is observed, discontinue use of this antimicrobial and initiate appropriate therapy.
Dogs
The safety of ceftiofur has not been determined for dogs intended for breeding, or pregnant dogs.
CEFTIFLEX ADVERSE REACTIONS
The use of ceftiofur may result in some sign of immediate and transient local pain to the animal.
CLINCAL MICROBIOLOGY
Summaries of MIC data are presented in Tables 1 and 2. Testing followed Clinical and Laboratory Standards Institute (CLSI) Guidelines.
| Animal
|
Organism
|
Number
Tested
|
Date
Tested
|
MIC9
0
(
µg
/
mL
)
 |
MIC
Range
(
µg
/
mL
)
|
| Bovine |
Mannheimia
haemolytica
|
461 |
1988-1992 |
0.06 |
≤ 0.03 - 0.13 |
|
|
Mannheimia
haemolytica
|
42 |
1993 |
0.015 |
≤ 0.003 - 0.03 |
|
|
Pasteurella
multocida
|
318 |
1988-1992 |
0.06 |
≤ 0.03 - 0.25 |
|
|
Pasteurella
multocida
|
48 |
1993 |
≤ 0.003 |
≤ 0.003 - 0.015 |
|
|
Histophilus
somni
|
109 |
1088-1992 |
0.06 |
≤ 0.03 - 0.13 |
|
|
Histophilus
somni
|
59 |
1993 |
≤ 0.0019 |
No range |
|
|
Fusobacterium
necrophorum
|
17 |
1994 |
≤ 0.06 |
No range |
| Swine |
Actinobacillus
pleuropn
.
|
83 |
1993 |
≤ 0.03 |
≤ 0.03 - 0.06 |
|
|
Pasteurella
multocida
|
74 |
1993 |
≤ 0.03 |
≤ 0.03 - 0.06 |
|
|
Streptococcus
suis
|
94 |
1993 |
0.25 |
≤ 0.03 – 1.0 |
|
|
Salmonella
choleraesuis
|
50 |
1993 |
1.0 |
1.0-2.0 |
|
|
β
-
hemolytic
Streptococcus spp |
24 |
1993 |
≤ 0.03 |
≤ 0.03 - 0.06 |
|
|
Actinobacillus
suis
|
77 |
1998 |
0.0078 |
0.0019-0.0078 |
|
|
Haemophilus
parasuis
|
76 |
1998 |
0.06 |
0.0039-0.25 |
| Sheep |
Mannheimia
haemolytica
|
39 |
1992 |
0.13 |
≤ 0.03 - 0.13 |
|
|
Pasteurella
multocida
|
23 |
1992 |
≤ 0.03 |
No range |
| Canine |
Escherichia
coli
|
44 |
1992 |
4.0 |
0.06-64.0 |
|
|
Escherichia
coli
|
18 |
1990 |
0.25 |
0.13-0.5 |
|
|
Proteus
mirabilis
|
17 |
1990 |
≤ 0.06 |
≤ 0.06- 0.5 |
|
|
Proteus
mirabilis
|
23 |
1992 |
1.0 |
≤ 0.06- 4.0 |
| Turkey |
Escherichia
coli
|
1204 |
1995 |
1.0 |
0.13->32.0 |
| Animal
|
Organism
|
Number
Tested
|
Date
Tested
|
MIC9
0
(
µg
/
mL
)**
|
MIC
Range
(
µg
/
mL
)
|
|
*The following in vitro data are available but their clinical significance is unknown. |
|||||
|
** Minimum inhibitory concentration (MIC) for 90% of the isolates. |
|||||
|
*** MIC5
0 is 32 µg/mL |
|||||
| Bovine |
Mannheimia
haemolytica
|
110 |
1997-1998 |
0.06 |
≤ 0.03 - 0.25 |
|
|
Mannheimia
haemolytica
|
139 |
1998-1999 |
≤ 0.03 |
≤ 0.03 - 0.5 |
|
|
Mannheimia
haemolytica
|
209 |
1999-2000 |
≤ 0.03 |
≤ 0.03 - 0.12 |
|
|
Mannheimia
haemolytica
|
189 |
2000-2001 |
≤ 0.03 |
≤ 0.03 - 0.12 |
|
|
Pasteurella
multocida
|
107 |
1997-1998 |
≤ 0.03 |
≤ 0.03 -0.25 |
|
|
Pasteurella
multocida
|
181 |
1998-1999 |
≤ 0.03 |
≤ 0.03 -0.5 |
|
|
Pasteurella
multocida
|
208 |
1999-2000 |
≤ 0.03 |
≤ 0.03 -0.12 |
|
|
Pasteurella
multocida
|
259 |
2000-2001 |
≤ 0.03 |
≤ 0.03 -0.12 |
|
|
Histophilus
somni
|
48 |
1997-1998 |
≤ 0.03 |
≤ 0.03-0.25 |
|
|
Histophilus
somni
|
87 |
1998-1999 |
≤ 0.03 |
≤ 0.03 -0.125 |
|
|
Histophilus
somni
|
77 |
1999-2000 |
≤ 0.03 |
≤ 0.03 -0.06 |
|
|
Histophilus
somni
|
129 |
2000-2001 |
≤ 0.03 |
≤ 0.03 -0.12 |
|
|
Bacteroides
fragilis
group
|
29 |
1994 |
16.0 |
≤ 0.06 ->16.0 |
|
|
Bacteroides
spp
.
non
-
fragilis
group
|
12 |
1994 |
16.0 |
0.13->16.0 |
|
|
Peptostreptocuccus
anaerobius
|
12 |
1994 |
2.0 |
0.13-2.0 |
| Swine |
Actinobacillus
pleuropn
.
|
97 |
1997-1998 |
≤ 0.03 |
No range |
|
|
Actinobacillus
pleuropn
.
|
111 |
1998-1999 |
≤ 0.03 |
≤ 0.03 - 0.25 |
|
|
Actinobacillus
pleuropn
.
|
126 |
1999-2000 |
≤ 0.03 |
≤ 0.03-0.06 |
|
|
Actinobacillus
pleuropn
.
|
89 |
2000 - 2001 |
≤ 0.03 |
≤ 0.03 -0.06 |
|
|
Pasteurella
multocida
|
114 |
1997-1998 |
≤ 0.03 |
≤ 0.03 -1.0 |
|
|
Pasteurella
multocida
|
147 |
1998-1999 |
≤ 0.03 |
≤ 0.03 -0.5 |
|
|
Pasteurella
multocida
|
173 |
1999-2000 |
≤ 0.03 |
≤ 0.03 -0.06 |
|
|
Pasteurella
multocida
|
186 |
2000-2001 |
≤ 0.03 |
≤ 0.03 -0.12 |
|
|
Streptococcus
suis
|
106 |
1997-1998 |
0.50 |
≤ 0.03 -4.0 |
|
|
Streptococcus
suis
|
142 |
1998-1999 |
0.25 |
≤ 0.03 -1.0 |
|
|
Streptococcus
suis
|
146 |
1999-2000 |
0.06 |
≤ 0.03 -4.0 |
|
|
Streptococcus
suis
|
167 |
2000-2001 |
0.06 |
≤ 0.03 -4.0 |
|
|
Salmonella
choleraesuis
|
96 |
1999-2000 |
1.0 |
0.03->4.0 |
|
|
Salmonella
choleraesuis
|
101 |
2000-2001 |
1.0 |
0.5-2.0 |
| Equine |
Streptococcus
equi
subsp
.
equi
.
|
12 |
1994 |
≤ 0.0019 |
No range |
|
|
Streptococcus
equi
subsp
.
equi
.
|
29 |
2002 |
≤ 0.03 |
≤ 0.03 -0.5 |
|
|
Streptococcus
zooepidemicus
|
48 |
1994 |
≤ 0.0019 |
No range |
|
|
Streptococcus
zooepidemicus
|
59 |
2002 |
≤ 0.03 |
≤ 0.03 -0.25 |
|
|
Rhodococcus
equi
|
66 |
1998 |
4.0 |
≤ 0.03 -16.0 |
|
|
Rhodococcus
equi
|
42 |
2002 |
8.0 |
≤ 0.03 ->32.0 |
|
|
Bacteroides
fragilis
group
|
32 |
1995 |
>16.0 |
≤ 0.03 ->16.0 |
|
|
Bacteroides
spp
.
nonfragilis
group
|
12 |
1995 |
4.0 |
0.25 - 4.0 |
|
|
Fusobacterium
necrophorum
|
16 |
1995 |
≤ 0.06 |
No range |
| Canine |
Escherichia
coli
|
26 |
2000 |
32.00 |
0.25->32 |
|
|
Proteus
mirabilis
|
14 |
2000 |
0.25 |
0.06-0.25 |
| Turkey |
Escherichia
coli
|
17 |
1998-1999 |
1.0 |
0.25-1.0 |
|
|
Escherichia
coli
|
25 |
1999-2000 |
0.50 |
0.25-1.0 |
|
|
Escherichia
coli
|
20 |
2000-20001 |
2.0 |
0.12-16.0 |
|
|
Citrobacter
spp
.
|
37 |
1995 |
32.0 |
0.5->32.0 |
|
|
Enterobacter
spp
.
|
51 |
1995 |
>32.0 |
0.13->32.0 |
|
|
Klebsiella
spp
.
|
100 |
1995 |
1.0 |
0.13 – 2.0 |
|
|
Proteus
spp
.
|
19 |
1995 |
1.0 |
0.06 – 32.0 |
|
|
Pseudomonas
spp
.***
|
31 |
1995 |
>32.0 |
0.06 - >32.0 |
|
|
Salmonella
spp
.
|
24 |
1995 |
1.0 |
0.5 – 1.0 |
|
|
Staphylococcus
spp .( coagulase negative ) |
17 |
1995 |
2.0 |
1.0 – 2.0 |
|
|
Staphylococcus
spp
.
( coagulase positive ) |
26 |
1995 |
8.0 |
0.13 - >32.0 |
| Chicken |
Escherichia
coli
|
62 |
1997-1998 |
0.50 |
0.25 -2.0 |
|
|
Escherichia
coli
|
53 |
1998-1999 |
4.0 |
0.25 ->4.0 |
|
|
Escherichia
coli
|
67 |
1999-2000 |
0.50 |
0.12 – 16.0 |
|
|
Escherichia
coli
|
90 |
2000-20001 |
1.0 |
≤ 0.03 -8.0 |
Based on the pharmacokinetic studies of ceftiofur in swine and cattle after a single intramuscular injection of 1.36 to 2.27 mg ceftiofur equivalents/lb (3.0 to 5.0 mg/kg) BW (swine) or 0.5 to 1.0 mg ceftiofur equivalents/ lb (1.1 to 2.2 mg/kg) BW (cattle) and the MIC and disk (30 ug) diffusion data, the following breakpoints are recommended by CLSI.
Zone Diameter (mm) MIC (µg/mL) Interpretation
≥ 21 ≤2.0 (S) Susceptible
18-20 4.0 (I) Intermediate
≤ 17 ≥ 8.0 (R) Resistant
A report of "Susceptible" indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of "Intermediate" is a technical buffer zone and isolates falling into this category should be retested. Alternatively the organism may be successfully treated if the infection is in a body site where drug is physiologically concentrated. A report of "Resistant" indicates that the achievable drug concentrations are unlikely to be inhibitory and other therapy should be selected.
Based on the pharmacokinetic studies of ceftiofur in horses after a single intramuscular injection of 1 mg ceftiofur equivalents/lb (2.2 mg/kg) BW, clinical effectiveness data and MIC data, the following breakpoint is recommended by CLSI.
Zone Diameter (mm) MIC (µg/mL) Interpretation
≥ 22 ≤ 0.25 (S) Susceptible
The susceptible only category is used for populations of organisms (usually one species) for which regression analysis (disk vs. MIC) cannot be performed. These breakpoints will permit detection of strains with decreased susceptibility as compared to the original population.
Standardized procedures require the use of laboratory control organisms for both standardized diffusion techniques and standardized dilution techniques. The 30 µg ceftiofur sodium disk should give the following zone diameters and the ceftiofur sodium standard reference powder (or disk) should provide the following MIC values for the reference strain. Ceftiofur sodium disks or powder reference standard is appropriate for both ceftiofur salts.
|
Organism
Name
(
ATCC
Number
)
|
Zone
Diameter
*
(
mm
)
|
MIC
Range
(
µg
/
mL
)
|
|
*All testing performed using a 30 µg disk. |
||
|
** Quality control ranges are applicable only to tests performed by disk diffusion test using a chocolate Mueller-Hinton agar, incubated in 5-7% CO2 for 20-24 hours. |
||
|
*** MIC quality control ranges are applicable only to tests performed by broth microdilution procedures using |
||
|
Veterinary fastidious medium (VFM). |
||
|
Escherichia
coli (25922) |
26-31 |
0.25-1.0 |
|
Staphylococcus
aureus (29213) |
- |
0.25-1.0 |
|
Staphylococcus
aureus (25923) |
27-31 |
- |
|
Pseudomonas
aeruginosa (27853) |
14-18 |
16.0-64.0 |
|
Actinobacillus
pleuropneumoniae (27090) |
34-42** |
0.004-0.015*** |
|
Histophilus
somni (700025) |
36-46** |
0.0005-0.004*** |
ANIMAL SAFETY
Cattle
Results from five-day tolerance study in normal feeder calves indicated that formulated ceftiofur was well tolerated at 25 times (25 mg/lb/day) the highest recommended dose of 1.0 mg/lb/day for five consecutive days. Ceftiofur administered intramuscularly had no adverse systemic effects.
In a 15-day safety/toxicity study, five steer and five heifer calves per group were intramuscularly administered formulated ceftiofur at 0 (vehicle control), 1, 3, 5 and 10 times the highest recommended dose of 1.0 mg/lb/day to determine the safety factor. There were no adverse systemic effects indicating that the formulated ceftiofur has a wide margin of safety when injected intramuscularly into the feeder calves at 10 times (10 mg/lb/day) the recommended dose for three times (15 days) the recommended three to five days of therapy. The formulation was shown to be a slight muscle irritant based on results of histopathological evaluation of the injection sites at 1 and 3 times the highest recommended dose of 1.0 mg/lb/day. The histopathological evaluations were conducted at posttreatment days 1, 3, 7 and 14.
The injection of Ceftiofur Sodium Sterile Powder at the recommended dose administered SC in the neck of cattle was well tolerated. However, a several square centimeter area of yellow-red discoloration resulting from a single SC injection persisted in many of the cattle beyond 4.5 days post-injection. Also, one of the animals developed an abscess at the injection site.
Swine
Result from a five-day tolerance study in normal feeder pigs indicated that formulated ceftiofur was well tolerated when administered at 57 mg/lb (more than 25 times the highest recommended daily dosage of 2.27 mg/lb of body weight) for five consecutive days. Ceftiofur administered intramuscularly to pigs produced no overt adverse signs of toxicity.
To determine the safety factor and to measure the muscle irritancy potential in swine, a safety/toxicity study was conducted. Five barrows and five gilts per group were intramuscularly administered formulated ceftiofur at 0, 2.27, 6.81 and 11.36 mg/lb of body weight for 15 days which is 0, 1, 3 and 5 times the highest recommended dose of 2.27 mg/lb of body weight/day and 5 times the recommended treatment length of 3 days. There were no adverse systemic effects indicating that formulated ceftiofur has a wide margin of safety when injected intramuscularly into feeder pigs at the highest recommended dose of 2.27 mg/lb/day for 3 days or at levels up to 5 times the highest recommended dose for 5 times the recommended length of treatment. The formulation was shown to be a slight muscle irritant based on results of histopathological evaluation of the injection sites at posttreatment days 1, 2, 3 and 4. By day 10 post injection the muscle reaction was subsiding and at day 15 post injection there was little evidence of muscle damage in any of the pigs in any of the treatment groups.
Sheep
In a 15-day safety/toxicity study in sheep, three wether, and three ewe lambs per group were given formulated ceftiofur sodium by the intramuscular route 0 (sterile water vehicle), 1, 3 or 5 times the recommended dose of 1.0 mg/lb/day for 3 times the recommended maximum duration of 5 days of treatment. There were no adverse systemic effects indicating that formulated ceftiofur is well tolerated and has a wide margin of safety in sheep. Based on examination of injection sites from study days 9, 11, 13, and 15, a low incidence of visual changes and histopathological findings of mild, reversible inflammation from all groups including the controls indicated that the formulation is a slight muscle irritant.
Goats
In a 15-day safety/toxicity study 5 lactating does, 5 dry does, and 5 wethers were given formulated ceftiofur by the intramuscular route with 11 mg/kg/day for 15 days. This constitutes 5 times the recommended dose for 3 times the recommended maximum duration of 5 days of treatment. There were no adverse systemic effects indicating that formulated ceftiofur is well tolerated and has a wide margin of safety in goats.
Horses
In a safety study, horses received a daily intramuscular injection of either 0 mg/lb/day (saline control), 1.0 mg/lb/day (50 mg/mL), 3.0 mg/lb/day (100 mg/mL), or 5.0 mg/lb/day (200 mg/mL) of an aqueous solution of ceftiofur sodium for 30 or 31 days. Ceftiofur sodium was well tolerated when administered intramuscularly to male and female horses at doses up to 5.0 mg/lb/day for 30 or 31 days. No clinical evidence of irritation was noted at any dose. The drug-related changes detected in this study were limited to a transient decrease in food consumption in horses receiving 3.0 or 5.0 mg/lb/day ceftiofur, and general mild skeletal muscle irritation at the injection sites which resolved by regeneration of muscle fibers.
In a tolerance study, horses received a single daily intravenous infusion of either 0 (saline), 10.0 or 25.0 mg/lb/day of an aqueous solution (50 mg/mL) of ceftiofur for 10 days. The results indicated that ceftiofur administered intravenously at a dose of 10.0 or 25.0 mg/lb/day apparently can change the bacterial flora of the large intestine thereby leading to inflammation of the large intestine with subsequent diarrhea and other clinical signs (loose feces, eating bedding straw, dehydration, rolling or colic and a dull, inactive demeanor). Decreased food consumption, a loss of body weight, hematologic changes related to acute inflammation and stress, and serum chemistry changes related to decreased food consumption and diarrhea were also associated with treatment at these doses. The adverse effects were most severe a few days after dosing was initiated and tended to become less severe toward the end of the 10-day dosing period.
Dogs
Ceftiofur sodium was well tolerated at the therapeutic dose and is safe for the treatment of urinary tract infections in dogs. In the acute safety study, ceftiofur was well tolerated by dogs at the recommended level (1.0 mg/lb) for 5-14 days. When administered subcutaneously for 42 consecutive days, one of four females developed thrombocytopenia (15 days) and anemia (36 days). Thrombocytopenia and anemia also occurred at the 3X and 5X dose levels. In the reversibility phase of the study (5X dose), the thrombocytopenia reversed within 8 days, and of the two anemic animals the male recovered within 6 weeks and the female was sacrificed due to the severity of the anemia.
In the 15-day tolerance study in dogs, high subcutaneous doses (25 and 125 times the recommended therapeutic dose) produced a progressive and dose-related thrombocytopenia, with some dogs also exhibiting anemia and bone marrow changes. The hematopoietic changes noted in dogs treated with ceftiofur were similar to those associated with long-term cephalosporin administration in dogs and also man. The hematopoietic effects are not expected to occur as a result of recommended therapy.
Day-Old Chicks
In acute toxicity study of ceftiofur in day-old chicks, a total of 60 male and 60 female chicks were each given single subcutaneous injections of 10, 100 or 1,000 mg/kg of body weight. Treatment on day 1 was followed by 6 days of observation; body weight was determined on days 1, 4 and 7; and selected hematology parameters were evaluated on day 4. No meaningful differences were noted among the treated and control groups of chicks for the parameters evaluated. Histopathologic evaluation of all deaths and chicks surviving to termination did not reveal a target organ or tissue of potential toxicity of ceftiofur when administered at up to 20 times (100 mg/kg) the intended highest use dosage.
Day-Old Turkey Poults
In an acute toxicity study of ceftiofur in day-old turkey poults, a total of 30 male and 30 female poults were each administered single subcutaneous injections of 100, 400 or 800 mg/kg body weight. Injection on day 1 was followed by 6 days of observation; body weight on days 1, 4, and 7; and selected hematology parameters on day 4. No meaningful differences were noted between the treated groups at 100 or 400 mg ceftiofur/ kg and a negative control group for the parameters evaluated. Histopathologic evaluation of all deaths and poults surviving to termination did not reveal a target organ or tissue of potential toxicity of ceftiofur when administered at up to 50 times (400 mg/kg) the highest use dosage. A dose of 800 mg/kg (100 times the intended highest use dosage) was toxic, resulting in clinical signs and deaths accompanied by gross and microscopic morphologic tissue alterations.
TISSUE RESIDUE DEPLETION
Cattle
A radiolabeled residue metabolism study established tolerances for ceftiofur residues in cattle kidney, liver and muscle. These tolerances of ceftiofur residues are 0.4 ppm in kidney, 2.0 ppm in liver, 1.0 ppm in muscle, and 0.1 ppm in milk.
A pivotal tissue residue decline study was conducted in cattle. In this study, cattle received an intramuscular injection of 1.0 mg of ceftiofur per lb body weight (2.2 mg of ceftiofur per kg body weight) for five consecutive days. Ceftiofur residues in tissues were less than the tolerances for ceftiofur residues in tissues such as kidney, liver and muscle by 4 days after dosing. These data collectively support a 4-day pre-slaughter withdrawal period in cattle when used according to label directions.
Swine
Radiolabeled residue metabolism studies established tolerances for ceftiofur residues in swine kidney, liver, and muscle. These tolerances of ceftiofur residues are 0.25 ppm in kidney, 3.0 ppm in liver and 2.0 ppm in muscle.
A pivotal tissue residue decline study was conducted in swine. In this study, pigs received 2.27 mg of ceftiofur per lb body weight (5 mg of ceftiofur per kg body weight) per day for three consecutive days. Ceftiofur residues in tissues were less than the tolerances for ceftiofur residues in tissues such as kidney, liver and muscle by 4 days after dosing. These data collectively support a 4-day pre-slaughter withdrawal period in swine when used according to label directions.
STORAGE CONDITIONS
Store unreconstituted product at controlled room temperature 20° to 25° C (68° to 77° F) [see USP].
Store reconstituted product either in a refrigerator 2° to 8° C (36° to 46° F) for up to 7 days or at controlled room temperature 20° to 25° C (68° to 77° F) [see USP] for up to 12 hours. Protect from light. Color may vary from off-white to a tan color. Color does not affect potency.
ONE-TIME SALVAGE PROCEDURE FOR RECONSTITUTED PRODUCT
At the end of the 7-day refrigeration or 12-hour room temperature storage period following reconstitution, any remaining reconstituted product may be frozen for up to 8 weeks without loss in potency or other chemical properties. This is a one-time only salvage procedure for the remaining product. To use this salvaged product at any time during the 8-week storage period, hold the vial under warm running water, gently swirling the container to accelerate thawing, or allow the frozen material to thaw at room temperature. Rapid freezing or thawing may result in vial breakage. Any product not used immediately upon thawing should be discarded.
HOW SUPPLIED
Ceftiofur Sodium Sterile Powder is available in the following package sizes:
1 gram vial
4 gram vial
1. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard - Second Edition. NCCLS document M31-A2. CLSI, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898, 2002.
ANADA 200-420, Approved by FDA
Mfd for: Med-Pharmex Inc. By: Cephazone Pharma LLC
Pomona, CA 91767 Pomona, CA 91767
Revised May 2009
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


CeftiflexCeftiofur Sodium INJECTION, POWDER, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||