Chantix
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CHANTIX safely and effectively. See full prescribing information for CHANTIX. CHANTIX® (varenicline) Tablets Initial U.S. Approval: 2006RECENT MAJOR CHANGES Boxed Warning 7/2009 Contraindications 4/2010 Known Hypersensitivity (4) Warnings and Precautions 7/2009 Neuropsychiatric Symptoms and Suicidality (5.1), Angioedema and Hypersensitivity Reactions (5.2), Serious Skin Reactions (5.3), Accidental Injury (5.4) BOXED WARNING WARNING: SERIOUS NEUROPSYCHIATRIC EVENTS See full prescribing information for complete boxed warning. Serious neuropsychiatric events have been reported in patients taking CHANTIX. (5.1 and 6.2) Advise patients and caregivers that the patient should stop taking CHANTIX and contact a healthcare provider immediately if agitation, hostility, depressed mood, or changes in behavior or thinking that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior while taking CHANTIX or shortly after discontinuing CHANTIX. (5.1 and 6.2) Weigh the risks of CHANTIX against benefits of its use. CHANTIX has been demonstrated to increase the likelihood of abstinence from smoking for as long as one year compared to treatment with placebo. The health benefits of quitting smoking are immediate and substantial. (5.1 and 6.2) INDICATIONS AND USAGECHANTIX is a nicotinic receptor partial agonist indicated for use as an aid to smoking cessation treatment. (1 and 2.1)DOSAGE AND ADMINISTRATION Begin CHANTIX dosing one week before the date set by the patient to stop smoking. (2.1) Starting week: 0.5 mg once daily on days 1–3 and 0.5 mg twice daily on days 4–7. (2.1) Continuing weeks: 1 mg twice daily for a total of 12 weeks. (2.1) An additional 12 weeks of treatment is recommended for successful quitters to increase likelihood of long-term abstinence. (2.1) Renal impairment: Reduce the dose in patients with severe renal impairment (estimated creatinine clearance 5% and twice the rate seen in placebo-treated patients) were nausea, abnormal (e.g., vivid, unusual, or strange) dreams, constipation, flatulence, and vomiting. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Other smoking cessation therapies: Safety and efficacy in combination with other smoking cessation therapies has not been established. Coadministration of varenicline and transdermal nicotine resulted in a high rate of discontinuation due to adverse events. (7.1) Effect of smoking cessation: Pharmacokinetics or pharmacodynamics of certain drugs may be altered due to smoking cessation with CHANTIX, necessitating dose adjustment. (7.2) USE IN SPECIFIC POPULATIONS Pregnancy: CHANTIX should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (8.1) Nursing Mothers: Discontinue drug or nursing taking into consideration importance of drug to mother (8.3) Pediatric Use: Safety and effectiveness not established (8.4) Renal Impairment: Dosage adjustment is required for severe renal impairment (2.2, 8.6)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 CHANTIX INDICATIONS AND USAGE
- 2 CHANTIX DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CHANTIX CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 CHANTIX ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 CHANTIX DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- 17.1 Set Quit Date and Continue to Attempt to Quit if Lapse
- 17.2 How To Take
- 17.3 Starting Week Dosage
- 17.4 Continuing Weeks Dosage
- 17.5 Dosage Adjustment for CHANTIX or Other Drugs
- 17.6 Counseling and Support
- 17.7 Neuropsychiatric Symptoms
- 17.8 History of Psychiatric Illness
- 17.9 Nicotine Withdrawal
- 17.10 Angioedema
- 17.11 Serious Skin Reactions
- 17.12 Driving or Operating Machinery
- 17.13 Vivid, Unusual, or Strange Dreams
- 17.14 Pregnancy and Lactation
FULL PRESCRIBING INFORMATION
Serious neuropsychiatric events including, but not limited to, depression, suicidal ideation, suicide attempt and completed suicide have been reported in patients taking CHANTIX. Some reported cases may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking. Depressed mood may be a symptom of nicotine withdrawal. Depression, rarely including suicidal ideation, has been reported in smokers undergoing a smoking cessation attempt without medication. However, some of these symptoms have occurred in patients taking CHANTIX who continued to smoke.
All patients being treated with CHANTIX should be observed for neuropsychiatric symptoms including changes in behavior, hostility, agitation, depressed mood, and suicide-related events, including ideation, behavior, and attempted suicide. These symptoms, as well as worsening of pre-existing psychiatric illness and completed suicide, have been reported in some patients attempting to quit smoking while taking CHANTIX in the postmarketing experience. When symptoms were reported, most were during CHANTIX treatment, but some were following discontinuation of CHANTIX therapy.
These events have occurred in patients with and without pre-existing psychiatric disease. Patients with serious psychiatric illness such as schizophrenia, bipolar disorder, and major depressive disorder did not participate in the premarketing studies of CHANTIX, and the safety and efficacy of CHANTIX in such patients has not been established.
Advise patients and caregivers that the patient should stop taking CHANTIX and contact a healthcare provider immediately if agitation, hostility, depressed mood, or changes in behavior or thinking that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. In many postmarketing cases, resolution of symptoms after discontinuation of CHANTIX was reported, although in some cases the symptoms persisted; therefore, ongoing monitoring and supportive care should be provided until symptoms resolve.
The risks of CHANTIX should be weighed against the benefits of its use. CHANTIX has been demonstrated to increase the likelihood of abstinence from smoking for as long as one year compared to treatment with placebo. The health benefits of quitting smoking are immediate and substantial. [see Warnings and Precautions (5.1) and Adverse Reactions 6.2)]
1 INDICATIONS AND USAGE
CHANTIX is indicated for use as an aid to smoking cessation treatment.
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dosage for Adults
Smoking cessation therapies are more likely to succeed for patients who are motivated to stop smoking and who are provided additional advice and support. Provide patients with appropriate educational materials and counseling to support the quit attempt.
The patient should set a date to stop smoking. Begin CHANTIX dosing one week before this date.
CHANTIX should be taken after eating and with a full glass of water.
The recommended dose of CHANTIX is 1 mg twice daily following a 1-week titration as follows:
| Days 1 – 3: | 0.5 mg once daily |
| Days 4 – 7: | 0.5 mg twice daily |
| Day 8 – end of treatment: | 1 mg twice daily |
Patients should be treated with CHANTIX for 12 weeks. For patients who have successfully stopped smoking at the end of 12 weeks, an additional course of 12 weeks' treatment with CHANTIX is recommended to further increase the likelihood of long-term abstinence.
Patients who do not succeed in stopping smoking during 12 weeks of initial therapy, or who relapse after treatment, should be encouraged to make another attempt once factors contributing to the failed attempt have been identified and addressed.
Consider a temporary or permanent dose reduction in patients who cannot tolerate the adverse effects of CHANTIX.
2.2 Dosage in Special Populations
Patients with Impaired Renal Function: No dosage adjustment is necessary for patients with mild to moderate renal impairment. For patients with severe renal impairment (estimated creatinine clearance <30 mL/min), the recommended starting dose of CHANTIX is 0.5 mg once daily. The dose may then be titrated as needed to a maximum dose of 0.5 mg twice a day. For patients with end-stage renal disease undergoing hemodialysis, a maximum dose of 0.5 mg once daily may be administered if tolerated [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Elderly and Patients with Impaired Hepatic Function: No dosage adjustment is necessary for patients with hepatic impairment. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Use in Specific Populations (8.5)].
3 DOSAGE FORMS AND STRENGTHS
Capsular, biconvex tablets: 0.5 mg (white to off-white, debossed with "Pfizer" on one side and "CHX 0.5" on the other side) and 1 mg (light blue, debossed with "Pfizer" on one side and "CHX 1.0" on the other side)
4 CONTRAINDICATIONS
CHANTIX is contraindicated in patients with a known history of serious hypersensitivity reactions or skin reactions to CHANTIX
5 WARNINGS AND PRECAUTIONS
5.1 Neuropsychiatric Symptoms and Suicidality
Serious neuropsychiatric symptoms have been reported in patients being treated with CHANTIX [see Boxed Warning and Adverse Reactions (6.2)]. These postmarketing reports have included changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, hostility, agitation, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide. Some reported cases may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking. Depressed mood may be a symptom of nicotine withdrawal. Depression, rarely including suicidal ideation, has been reported in smokers undergoing a smoking cessation attempt without medication. However, some of these symptoms have occurred in patients taking CHANTIX who continued to smoke. When symptoms were reported, most were during CHANTIX treatment, but some were following discontinuation of CHANTIX therapy.
These events have occurred in patients with and without pre-existing psychiatric disease; some patients have experienced worsening of their psychiatric illnesses. All patients being treated with CHANTIX should be observed for neuropsychiatric symptoms or worsening of pre-existing psychiatric illness. Patients with serious psychiatric illness such as schizophrenia, bipolar disorder, and major depressive disorder did not participate in the premarketing studies of CHANTIX, and the safety and efficacy of CHANTIX in such patients has not been established.
Advise patients and caregivers that the patient should stop taking CHANTIX and contact a healthcare provider immediately if agitation, depressed mood, changes in behavior or thinking that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. In many postmarketing cases, resolution of symptoms after discontinuation of CHANTIX was reported, although in some cases the symptoms persisted, therefore, ongoing monitoring and supportive care should be provided until symptoms resolve.
The risks of CHANTIX should be weighed against the benefits of its use. CHANTIX has been demonstrated to increase the likelihood of abstinence from smoking for as long as one year compared to treatment with placebo. The health benefits of quitting smoking are immediate and substantial.
5.2 Angioedema and Hypersensitivity Reactions
There have been postmarketing reports of hypersensitivity reactions including angioedema in patients treated with CHANTIX [see Adverse Reactions (6.2), and Patient Counseling Information (17.10)]. Clinical signs included swelling of the face, mouth (tongue, lips, and gums), extremities, and neck (throat and larynx). There were infrequent reports of life-threatening angioedema requiring emergent medical attention due to respiratory compromise. Instruct patients to discontinue CHANTIX and immediately seek medical care if they experience these symptoms.
5.3 Serious Skin Reactions
There have been postmarketing reports of rare but serious skin reactions, including Stevens-Johnson Syndrome and erythema multiforme, in patients using CHANTIX [see Adverse Reactions (6.2)]. As these skin reactions can be life-threatening, instruct patients to stop taking CHANTIX and contact a healthcare provider immediately at the first appearance of a skin rash with mucosal lesions or any other signs of hypersensitivity.
5.4 Accidental Injury
There have been postmarketing reports of traffic accidents, near-miss incidents in traffic, or other accidental injuries in patients taking CHANTIX. In some cases, the patients reported somnolence, dizziness, loss of consciousness or difficulty concentrating that resulted in impairment, or concern about potential impairment, in driving or operating machinery. Advise patients to use caution driving or operating machinery or engaging in other potentially hazardous activities until they know how CHANTIX may affect them.
5.5 Nausea
Nausea was the most common adverse reaction reported with CHANTIX treatment. Nausea was generally described as mild or moderate and often transient; however, for some patients, it was persistent over several months. The incidence of nausea was dose-dependent. Initial dose-titration was beneficial in reducing the occurrence of nausea. For patients treated to the maximum recommended dose of 1 mg twice daily following initial dosage titration, the incidence of nausea was 30% compared with 10% in patients taking a comparable placebo regimen. In patients taking CHANTIX 0.5 mg twice daily following initial titration, the incidence was 16% compared with 11% for placebo. Approximately 3% of patients treated with CHANTIX 1 mg twice daily in studies involving 12 weeks of treatment discontinued treatment prematurely because of nausea. For patients with intolerable nausea, a dose reduction should be considered.
6 ADVERSE REACTIONS
The following serious adverse reactions were reported in postmarketing experience and are discussed in greater detail in other sections of the labeling:
- Neuropsychiatric symptoms and suicidality [see Boxed Warning and Warnings and Precautions (5.1)
- Angioedema and hypersensitivity reactions [see Warnings and Precautions (5.2)]
- Serious skin reactions [see Warnings and Precautions (5.3)]
- Accidental injury [see Warnings and Precautions (5.4)]
In the placebo-controlled studies, the most common adverse events associated with CHANTIX (>5% and twice the rate seen in placebo-treated patients) were nausea, abnormal (vivid, unusual, or strange) dreams, constipation, flatulence, and vomiting.
The treatment discontinuation rate due to adverse events in patients dosed with 1 mg twice daily was 12% for CHANTIX, compared to 10% for placebo in studies of three months' treatment. In this group, the discontinuation rates that are higher than placebo for the most common adverse events in CHANTIX-treated patients were as follows: nausea (3% vs. 0.5% for placebo), insomnia (1.2% vs. 1.1% for placebo), and abnormal dreams (0.3% vs. 0.2% for placebo).
Smoking cessation, with or without treatment, is associated with nicotine withdrawal symptoms and has also been associated with the exacerbation of underlying psychiatric illness.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reactions rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During the premarketing development of CHANTIX, over 4500 subjects were exposed to CHANTIX, with over 450 treated for at least 24 weeks and approximately 100 for a year. Most study participants were treated for 12 weeks or less.
The most common adverse event associated with CHANTIX treatment is nausea, occurring in 30% of patients treated at the recommended dose, compared with 10% in patients taking a comparable placebo regimen [see Warnings and Precautions (5.5)].
Table 1 shows the adverse events for CHANTIX and placebo in the 12-week fixed dose studies with titration in the first week [Studies 2 (titrated arm only), 4, and 5]. Adverse events were categorized using the Medical Dictionary for Regulatory Activities (MedDRA, Version 7.1).
MedDRA High Level Group Terms (HLGT) reported in ≥ 5% of patients in the CHANTIX 1 mg twice daily dose group, and more commonly than in the placebo group, are listed, along with subordinate Preferred Terms (PT) reported in ≥ 1% of CHANTIX patients (and at least 0.5% more frequent than placebo). Closely related Preferred Terms such as 'Insomnia', 'Initial insomnia', 'Middle insomnia', 'Early morning awakening' were grouped, but individual patients reporting two or more grouped events are only counted once.
| SYSTEM ORGAN CLASS High Level Group Term Preferred Term |
CHANTIX 0.5 mg BID N=129 |
CHANTIX 1 mg BID N=821 |
Placebo N=805 |
| GASTROINTESTINAL (GI) | |||
| GI Signs and Symptoms | |||
| Nausea | 16 | 30 | 10 |
Abdominal Pain  |
5 | 7 | 5 |
| Flatulence | 9 | 6 | 3 |
| Dyspepsia | 5 | 5 | 3 |
| Vomiting | 1 | 5 | 2 |
| GI Motility/Defecation Conditions | |||
| Constipation | 5 | 8 | 3 |
| Gastroesophageal reflux disease | 1 | 1 | 0 |
| Salivary Gland Conditions | |||
| Dry mouth | 4 | 6 | 4 |
| PSYCHIATRIC DISORDERS | |||
| Sleep Disorder/Disturbances |
|||
Insomnia  |
19 | 18 | 13 |
| Abnormal dreams | 9 | 13 | 5 |
| Sleep disorder | 2 | 5 | 3 |
| Nightmare | 2 | 1 | 0 |
| NERVOUS SYSTEM | |||
| Headaches | |||
| Headache | 19 | 15 | 13 |
| Neurological Disorders NEC |
|||
| Dysgeusia | 8 | 5 | 4 |
| Somnolence | 3 | 3 | 2 |
| Lethargy | 2 | 1 | 0 |
| GENERAL DISORDERS | |||
| General Disorders NEC | |||
| Fatigue/Malaise/Asthenia | 4 | 7 | 6 |
| RESPIR/THORACIC/MEDIAST | |||
| Respiratory Disorders NEC | |||
| Rhinorrhea | 0 | 1 | 0 |
| Dyspnea | 2 | 1 | 1 |
| Upper Respiratory Tract Disorder | 7 | 5 | 4 |
| SKIN/SUBCUTANEOUS TISSUE | |||
| Epidermal and Dermal Conditions | |||
| Rash | 1 | 3 | 2 |
| Pruritis | 0 | 1 | 1 |
| METABOLISM & NUTRITION | |||
| Appetite/General Nutrit. Disorders | |||
| Increased appetite | 4 | 3 | 2 |
| Decreased appetite/Anorexia | 1 | 2 | 1 |
The overall pattern and frequency of adverse events during the longer-term trials was similar to those described in Table 1, though several of the most common events were reported by a greater proportion of patients with long-term use (e.g., nausea was reported in 40% of patients treated with CHANTIX 1 mg twice daily in a one-year study, compared to 8% of placebo-treated patients).
Following is a list of treatment-emergent adverse events reported by patients treated with CHANTIX during all clinical trials. The listing does not include those events already listed in the previous tables or elsewhere in labeling, those events for which a drug cause was remote, those events which were so general as to be uninformative, and those events reported only once which did not have a substantial probability of being acutely life-threatening.
Blood and Lymphatic System Disorders. Infrequent: anemia, lymphadenopathy. Rare: leukocytosis, splenomegaly, thrombocytopenia.
Cardiac Disorders. Infrequent: angina pectoris, arrhythmia, bradycardia, myocardial infarction, palpitations, tachycardia, ventricular extrasystoles. Rare: acute coronary syndrome, atrial fibrillation, cardiac flutter, cor pulmonale, coronary artery disease.
Ear and Labyrinth Disorders. Infrequent: tinnitus, vertigo. Rare: deafness, Meniere's disease.
Endocrine Disorders. Infrequent: thyroid gland disorders.
Eye Disorders. Infrequent: conjunctivitis, dry eye, eye irritation, eye pain, vision blurred, visual disturbance. Rare: acquired night blindness, blindness transient, cataract subcapsular, ocular vascular disorder, photophobia, vitreous floaters
Gastrointestinal Disorders. Frequent: diarrhea. Infrequent: dysphagia, enterocolitis, eructation, esophagitis, gastritis, gastrointestinal hemorrhage, mouth ulceration. Rare: gastric ulcer, intestinal obstruction, pancreatitis acute.
General Disorders and Administration Site Conditions. Frequent: chest pain, edema, influenza-like illness. Infrequent: chest discomfort, chills, pyrexia.
Hepatobiliary Disorders. Infrequent: gall bladder disorder
Investigations. Frequent: liver function test abnormal, weight increased. Infrequent: electrocardiogram abnormal, muscle enzyme increased, urine analysis abnormal.
Metabolism and Nutrition Disorders. Infrequent: diabetes mellitus, hyperlipidemia, hypokalemia. Rare: hypoglycemia.
Musculoskeletal and Connective Tissue Disorders. Frequent: arthralgia, back pain, muscle cramp, musculoskeletal pain, myalgia. Infrequent: arthritis, osteoporosis. Rare: myositis.
Nervous System Disorders. Frequent: disturbance in attention, dizziness, sensory disturbance. Infrequent: amnesia, migraine, parosmia, psychomotor hyperactivity, restless legs syndrome, syncope, tremor. Rare: balance disorder, cerebrovascular accident, convulsion, dysarthria, facial palsy, mental impairment, multiple sclerosis, nystagmus, psychomotor skills impaired, transient ischemic attack, visual field defect.
Psychiatric Disorders. Infrequent: disorientation, dissociation, libido decreased, mood swings, thinking abnormal. Rare: bradyphrenia, euphoric mood.
Renal and Urinary Disorders. Frequent: polyuria. Infrequent: nephrolithiasis, nocturia, urethral syndrome, urine abnormality. Rare: renal failure acute, urinary retention.
Reproductive System and Breast Disorders. Rare: sexual dysfunction. Frequent: menstrual disorder. Infrequent: erectile dysfunction.
Respiratory, Thoracic and Mediastinal Disorders. Frequent: epistaxis, respiratory disorders. Infrequent: asthma. Rare: pleurisy, pulmonary embolism.
Skin and Subcutaneous Tissue Disorders. Frequent: hyperhidrosis. Infrequent: acne, dry skin, eczema, erythema, psoriasis, urticaria. Rare: photosensitivity reaction.
Vascular Disorders. Frequent: hot flush. Infrequent: thrombosis.
6.2 Postmarketing Experience
The following adverse events have been reported during post-approval use of CHANTIX. Because these events are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been reports of depression, mania, psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, hostility, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide in patients attempting to quit smoking while taking CHANTIX [see Boxed Warning, Warnings and Precautions (5.1)]. Smoking cessation with or without treatment is associated with nicotine withdrawal symptoms and the exacerbation of underlying psychiatric illness. Not all patients had known pre-existing psychiatric illness and not all had discontinued smoking.
There have been reports of hypersensitivity reactions, including angioedema [see Warnings and Precautions (5.2)].
There have also been reports of serious skin reactions, including Stevens-Johnson Syndrome and erythema multiforme, in patients taking CHANTIX [see Warnings and Precautions (5.3)].
7 DRUG INTERACTIONS
Based on varenicline characteristics and clinical experience to date, CHANTIX has no clinically meaningful pharmacokinetic drug interactions [see Clinical Pharmacology (12.3)].
7.1 Use With Other Drugs for Smoking Cessation
Safety and efficacy of CHANTIX in combination with other smoking cessation therapies have not been studied.
Bupropion : Varenicline (1 mg twice daily) did not alter the steady-state pharmacokinetics of bupropion (150 mg twice daily) in 46 smokers. The safety of the combination of bupropion and varenicline has not been established.
Nicotine replacement therapy (NRT ): Although co-administration of varenicline (1 mg twice daily) and transdermal nicotine (21 mg/day) for up to 12 days did not affect nicotine pharmacokinetics, the incidence of nausea, headache, vomiting, dizziness, dyspepsia, and fatigue was greater for the combination than for NRT alone. In this study, eight of twenty-two (36%) patients treated with the combination of varenicline and NRT prematurely discontinued treatment due to adverse events, compared to 1 of 17 (6%) of patients treated with NRT and placebo.
7.2 Effect of Smoking Cessation on Other Drugs
Physiological changes resulting from smoking cessation, with or without treatment with CHANTIX, may alter the pharmacokinetics or pharmacodynamics of certain drugs (e.g., theophylline, warfarin, insulin) for which dosage adjustment may be necessary.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
There are no adequate and well-controlled studies of CHANTIX use in pregnant women. In animal studies, CHANTIX caused decreased fetal weights, increased auditory startle response, and decreased fertility in offspring. CHANTIX should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In reproductive and developmental toxicity studies, pregnant rats and rabbits received varenicline succinate during organogenesis at oral doses up to 15 and 30 mg/kg/day, respectively. These exposures were 36 (rats) and 50 (rabbits) times the human exposure (based on AUC) at the maximum recommended human dose (MRHD) of 1 mg BID. While no fetal structural abnormalities occurred in either species, reduced fetal weights occurred in rabbits at the highest dose (exposures 50 times the human exposure at the MRHD based on AUC). Fetal weight reduction did not occur at animal exposures 23 times the human exposure at the MRHD based on AUC.
In a pre- and postnatal development study, pregnant rats received up to 15 mg/kg/day of oral varenicline succinate from organogenesis through lactation. These resulted in exposures up to 36 times the human exposure (based on AUC) at the MRHD of 1 mg BID. Decreased fertility and increased auditory startle response occurred in offspring.
8.3 Nursing Mothers
It is not known whether CHANTIX is excreted in human milk. In animal studies varenicline was excreted in milk of lactating animals. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from CHANTIX, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of CHANTIX in pediatric patients have not been established.
8.5 Geriatric Use
A combined single- and multiple-dose pharmacokinetic study demonstrated that the pharmacokinetics of 1 mg varenicline given once daily or twice daily to 16 healthy elderly male and female smokers (aged 65–75 yrs) for 7 consecutive days was similar to that of younger subjects. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Varenicline is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.2) ].
No dosage adjustment is recommended for elderly patients.
8.6 Renal Impairment
Varenicline is substantially eliminated by renal glomerular filtration along with active tubular secretion. Dose reduction is not required in patients with mild to moderate renal impairment. For patients with severe renal impairment (estimated creatinine clearance <30 mL/min), and for patients with end-stage renal disease undergoing hemodialysis, dosage adjustment is needed. [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Varenicline is not a controlled substance.
9.3 Dependence
Humans : Fewer than 1 out of 1000 patients reported euphoria in clinical trials with CHANTIX. At higher doses (greater than 2 mg), CHANTIX produced more frequent reports of gastrointestinal disturbances such as nausea and vomiting. There is no evidence of dose-escalation to maintain therapeutic effects in clinical studies, which suggests that tolerance does not develop. Abrupt discontinuation of CHANTIX was associated with an increase in irritability and sleep disturbances in up to 3% of patients. This suggests that, in some patients, varenicline may produce mild physical dependence which is not associated with addiction.
In a human laboratory abuse liability study, a single oral dose of 1 mg varenicline did not produce any significant positive or negative subjective responses in smokers. In non-smokers, 1 mg varenicline produced an increase in some positive subjective effects, but this was accompanied by an increase in negative adverse effects, especially nausea. A single oral dose of 3 mg varenicline uniformly produced unpleasant subjective responses in both smokers and non-smokers.
Animals : Studies in rodents have shown that varenicline produces behavioral responses similar to those produced by nicotine. In rats trained to discriminate nicotine from saline, varenicline produced full generalization to the nicotine cue. In self-administration studies, the degree to which varenicline substitutes for nicotine is dependent upon the requirement of the task. Rats trained to self-administer nicotine under easy conditions continued to self-administer varenicline to a degree comparable to that of nicotine; however in a more demanding task, rats self-administered varenicline to a lesser extent than nicotine. Varenicline pretreatment also reduced nicotine self-administration.
10 OVERDOSAGE
In case of overdose, standard supportive measures should be instituted as required.
Varenicline has been shown to be dialyzed in patients with end stage renal disease [see Clinical Pharmacology (12.3)], however, there is no experience in dialysis following overdose.
11 DESCRIPTION
CHANTIX tablets contain varenicline (as the tartrate salt), which is a partial agonist selective for α4β2 nicotinic acetylcholine receptor subtypes.
Varenicline, as the tartrate salt, is a powder which is a white to off-white to slightly yellow solid with the following chemical name: 7,8,9,10-tetrahydro-6,10-methano-6H-pyrazino[2,3- h][3]benzazepine, (2R,3R)-2,3-dihydroxybutanedioate (1:1). It is highly soluble in water. Varenicline tartrate has a molecular weight of 361.35 Daltons, and a molecular formula of C13H13N3 • C4H6O6. The chemical structure is:

CHANTIX is supplied for oral administration in two strengths: a 0.5 mg capsular biconvex, white to off-white, film-coated tablet debossed with "Pfizer" on one side and "CHX 0.5" on the other side and a 1 mg capsular biconvex, light blue film-coated tablet debossed with "Pfizer" on one side and "CHX 1.0" on the other side. Each 0.5 mg CHANTIX tablet contains 0.85 mg of varenicline tartrate equivalent to 0.5 mg of varenicline free base; each 1mg CHANTIX tablet contains 1.71 mg of varenicline tartrate equivalent to 1 mg of varenicline free base. The following inactive ingredients are included in the tablets: microcrystalline cellulose, anhydrous dibasic calcium phosphate, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, Opadry® White (for 0.5 mg), Opadry® Blue (for 1 mg), and Opadry® Clear.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Varenicline binds with high affinity and selectivity at α4β2 neuronal nicotinic acetylcholine receptors. The efficacy of CHANTIX in smoking cessation is believed to be the result of varenicline's activity at α4β2 sub-type of the nicotinic receptor where its binding produces agonist activity, while simultaneously preventing nicotine binding to these receptors.
Electrophysiology studies in vitro and neurochemical studies in vivo have shown that varenicline binds to α4β2 neuronal nicotinic acetylcholine receptors and stimulates receptor-mediated activity, but at a significantly lower level than nicotine. Varenicline blocks the ability of nicotine to activate α4β2 receptors and thus to stimulate the central nervous mesolimbic dopamine system, believed to be the neuronal mechanism underlying reinforcement and reward experienced upon smoking. Varenicline is highly selective and binds more potently to α4β2 receptors than to other common nicotinic receptors (>500-fold α3β4, >3500-fold α7, >20,000-fold α1βγδ), or to non-nicotinic receptors and transporters (>2000-fold). Varenicline also binds with moderate affinity (Ki = 350 nM) to the 5-HT3 receptor.
12.3 Pharmacokinetics
Absorption/Distribution : Maximum plasma concentrations of varenicline occur typically within 3–4 hours after oral administration. Following administration of multiple oral doses of varenicline, steady-state conditions were reached within 4 days. Over the recommended dosing range, varenicline exhibits linear pharmacokinetics after single or repeated doses. In a mass balance study, absorption of varenicline was virtually complete after oral administration and systemic availability was ~90%. Oral bioavailability of varenicline is unaffected by food or time-of-day dosing. Plasma protein binding of varenicline is low (≤20%) and independent of both age and renal function.
Metabolism/Elimination : The elimination half-life of varenicline is approximately 24 hours. Varenicline undergoes minimal metabolism, with 92% excreted unchanged in the urine. Renal elimination of varenicline is primarily through glomerular filtration along with active tubular secretion possibly via the organic cation transporter, OCT2.
Pharmacokinetics in Special Patient Populations : There are no clinically meaningful differences in varenicline pharmacokinetics due to age, race, gender, smoking status, or use of concomitant medications, as demonstrated in specific pharmacokinetic studies and in population pharmacokinetic analyses.
Renal Impairment: Varenicline pharmacokinetics were unchanged in subjects with mild renal impairment (estimated creatinine clearance >50 mL/min and ≤80 mL/min). In subjects with moderate renal impairment (estimated creatinine clearance ≥30 mL/min and ≤50 mL/min), varenicline exposure increased 1.5-fold compared with subjects with normal renal function (estimated creatinine clearance >80 mL/min). In subjects with severe renal impairment (estimated creatinine clearance <30 mL/min), varenicline exposure was increased 2.1-fold. In subjects with end-stage-renal disease (ESRD) undergoing a three-hour session of hemodialysis for three days a week, varenicline exposure was increased 2.7-fold following 0.5 mg once daily administration for 12 days. The plasma Cmax and AUC of varenicline noted in this setting were similar to those of healthy subjects receiving 1 mg twice daily. [see Dosage and Administration (2.2), and Use in Specific Populations (8.6)]. Additionally, in subjects with ESRD, varenicline was efficiently removed by hemodialysis [see Overdosage (10)].
Geriatric Patients: A combined single- and multiple-dose pharmacokinetic study demonstrated that the pharmacokinetics of 1 mg varenicline given once daily or twice daily to 16 healthy elderly male and female smokers (aged 65–75 yrs) for 7 consecutive days was similar to that of younger subjects.
Pediatric Patients: Because the safety and effectiveness of CHANTIX in pediatric patients have not been established, CHANTIX is not recommended for use in patients under 18 years of age. When 22 pediatric patients aged 12 to 17 years (inclusive) received a single 0.5 mg or 1 mg dose of varenicline, the pharmacokinetics of varenicline were approximately dose-proportional between the 0.5 mg and 1 mg doses. Systemic exposure, as assessed by AUC (0–∞), and renal clearance of varenicline were comparable to those of an adult population.
Hepatic Impairment: Due to the absence of significant hepatic metabolism, varenicline pharmacokinetics should be unaffected in patients with hepatic impairment.
Drug-Drug Interactions : Drug interaction studies were performed with varenicline and digoxin, warfarin, transdermal nicotine, bupropion, cimetidine, and metformin. No clinically meaningful pharmacokinetic drug-drug interactions have been identified.
In vitro studies demonstrated that varenicline does not inhibit the following cytochrome P450 enzymes (IC50 >6400 ng/mL): 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4/5. Also, in human hepatocytes in vitro, varenicline does not induce the cytochrome P450 enzymes 1A2 and 3A4.
In vitro studies demonstrated that varenicline does not inhibit human renal transport proteins at therapeutic concentrations. Therefore, drugs that are cleared by renal secretion (e.g., metformin [see below]) are unlikely to be affected by varenicline.
In vitro studies demonstrated the active renal secretion of varenicline is mediated by the human organic cation transporter OCT2. Co-administration with inhibitors of OCT2 (e.g., cimeditine [see below]) may not necessitate a dose adjustment of CHANTIX as the increase in systemic exposure to CHANTIX is not expected to be clinically meaningful. Furthermore, since metabolism of varenicline represents less than 10% of its clearance, drugs known to affect the cytochrome P450 system are unlikely to alter the pharmacokinetics of CHANTIX [ see Clinical Pharmacology (12.3)]; therefore, a dose adjustment of CHANTIX would not be required.
Metformin: When co-administered to 30 smokers, varenicline (1 mg twice daily) did not alter the steady-state pharmacokinetics of metformin (500 mg twice daily), which is a substrate of OCT2. Metformin had no effect on varenicline steady-state pharmacokinetics.
Cimetidine: Co-administration of an OCT2 inhibitor, cimetidine (300 mg four times daily), with varenicline (2 mg single dose) to 12 smokers increased the systemic exposure of varenicline by 29% (90% CI: 21.5%, 36.9%) due to a reduction in varenicline renal clearance.
Digoxin: Varenicline (1 mg twice daily) did not alter the steady-state pharmacokinetics of digoxin administered as a 0.25 mg daily dose in 18 smokers.
Warfarin: Varenicline (1 mg twice daily) did not alter the pharmacokinetics of a single 25 mg dose of (R, S)-warfarin in 24 smokers. Prothrombin time (INR) was not affected by varenicline. Smoking cessation itself may result in changes to warfarin pharmacokinetics [see Drug Interactions (7.2)].
Use with Other Drugs for Smoking Cessation:
Bupropion: Varenicline (1 mg twice daily) did not alter the steady-state pharmacokinetics of bupropion (150 mg twice daily) in 46 smokers [see Drug Interactions (7.1)].
Nicotine replacement therapy (NRT): Although co-administration of varenicline (1 mg twice daily) and transdermal nicotine (21 mg/day) for up to 12 days did not affect nicotine pharmacokinetics, the incidence of adverse reactions was greater for the combination than for NRT alone [see Drug Interactions (7.1) ].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis : Lifetime carcinogenicity studies were performed in CD-1 mice and Sprague-Dawley rats. There was no evidence of a carcinogenic effect in mice administered varenicline by oral gavage for 2 years at doses up to 20 mg/kg/day (47 times the maximum recommended human daily exposure based on AUC). Rats were administered varenicline (1, 5, and 15 mg/kg/day) by oral gavage for 2 years. In male rats (n = 65 per sex per dose group), incidences of hibernoma (tumor of the brown fat) were increased at the mid dose (1 tumor, 5 mg/kg/day, 23 times the maximum recommended human daily exposure based on AUC) and maximum dose (2 tumors, 15 mg/kg/day, 67 times the maximum recommended human daily exposure based on AUC). The clinical relevance of this finding to humans has not been established. There was no evidence of carcinogenicity in female rats.
Mutagenesis : Varenicline was not genotoxic, with or without metabolic activation, in the following assays: Ames bacterial mutation assay; mammalian CHO/HGPRT assay; and tests for cytogenetic aberrations in vivo in rat bone marrow and in vitro in human lymphocytes.
Impairment of Fertility : There was no evidence of impairment of fertility in either male or female Sprague-Dawley rats administered varenicline succinate up to 15 mg/kg/day (67 and 36 times, respectively, the maximum recommended human daily exposure based on AUC at 1 mg twice daily). However, a decrease in fertility was noted in the offspring of pregnant rats who were administered varenicline succinate at an oral dose of 15 mg/kg/day (36 times the maximum recommended human daily exposure based on AUC at 1 mg BID). This decrease in fertility in the offspring of treated female rats was not evident at an oral dose of 3 mg/kg/day (9 times the maximum recommended human daily exposure based on AUC at 1 mg twice daily).
14 CLINICAL STUDIES
The efficacy of CHANTIX in smoking cessation was demonstrated in six clinical trials in which a total of 3659 chronic cigarette smokers (≥10 cigarettes per day) were treated with CHANTIX. In all clinical studies, abstinence from smoking was determined by patient self-report and verified by measurement of exhaled carbon monoxide (CO≤10 ppm) at weekly visits. Among the CHANTIX-treated patients enrolled in these studies, the completion rate was 65%. Except for the dose-ranging study (Study 1) and the maintenance of abstinence study (Study 6), patients were treated for 12 weeks and then were followed for 40 weeks post-treatment. Most patients enrolled in these trials were white (79–96%). All studies enrolled almost equal numbers of men and women. The average age of patients in these studies was 43 years. Patients on average had smoked about 21 cigarettes per day for an average of approximately 25 years.
In all studies, patients were provided with an educational booklet on smoking cessation and received up to 10 minutes of smoking cessation counseling at each weekly treatment visit according to Agency for Healthcare Research and Quality guidelines. Patients set a date to stop smoking (target quit date [TQD]) with dosing starting 1 week before this date.
14.1 Initiation of Abstinence
Study 1 : This was a six-week dose-ranging study comparing CHANTIX to placebo. This study provided initial evidence that CHANTIX at a total dose of 1 mg per day or 2 mg per day was effective as an aid to smoking cessation.
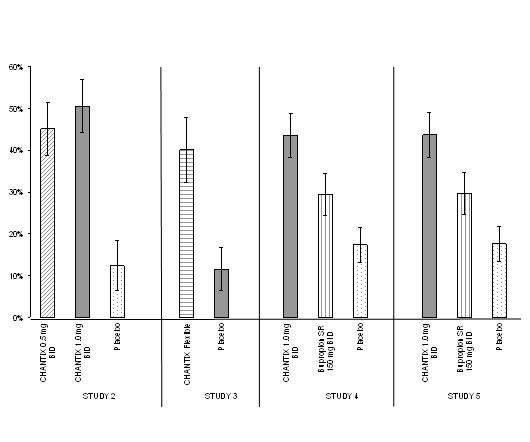
Study 2 : This study of 627 patients compared CHANTIX 1 mg per day and 2 mg per day with placebo. Patients were treated for 12 weeks (including one week titration) and then were followed for 40 weeks post-treatment. CHANTIX was given in two divided doses daily. Each dose of CHANTIX was given in two different regimens, with and without initial dose titration, to explore the effect of different dosing regimens on tolerability. For the titrated groups, dosage was titrated up over the course of one week, with full dosage achieved starting with the second week of dosing. The titrated and nontitrated groups were pooled for efficacy analysis.
Forty-five percent of patients receiving CHANTIX 1 mg per day (0.5 mg twice daily) and 51% of patients receiving 2 mg per day (1 mg twice daily) had CO-confirmed continuous abstinence during weeks 9 through 12 compared to 12% of patients in the placebo group (Figure 1). In addition, 31% of the 1 mg per day group and 31% of the 2 mg per day group were continuously abstinent from one week after TQD through the end of treatment as compared to 8% of the placebo group.
Study 3 : This flexible-dosing study of 312 patients examined the effect of a patient-directed dosing strategy of CHANTIX or placebo. After an initial one-week titration to a dose of 0.5 mg twice daily, patients could adjust their dosage as often as they wished between 0.5 mg once daily to 1 mg twice daily per day. Sixty-nine percent of patients titrated to the maximum allowable dose at any time during the study. For 44% of patients, the modal dose selected was 1 mg twice daily; for slightly over half of the study participants, the modal dose selected was 1 mg/day or less.
Of the patients treated with CHANTIX, 40% had CO-confirmed continuous abstinence during weeks 9 through 12 compared to 12% in the placebo group. In addition, 29% of the CHANTIX group were continuously abstinent from one week after TQD through the end of treatment as compared to 9% of the placebo group.
Study 4 and Study 5 : These identical double-blind studies compared CHANTIX 2 mg per day, bupropion sustained-release (SR) 150 mg twice daily, and placebo. Patients were treated for 12 weeks and then were followed for 40 weeks post-treatment. The CHANTIX dosage of 1 mg twice daily was achieved using a titration of 0.5 mg once daily for the initial 3 days followed by 0.5 mg twice daily for the next 4 days. The bupropion SR dosage of 150 mg twice daily was achieved using a 3-day titration of 150 mg once daily. Study 4 enrolled 1022 patients and Study 5 enrolled 1023 patients. Patients inappropriate for bupropion treatment or patients who had previously used bupropion were excluded.
In Study 4, patients treated with CHANTIX had a superior rate of CO-confirmed abstinence during weeks 9 through 12 (44%) compared to patients treated with bupropion SR (30%) or placebo (17%). The bupropion SR quit rate was also superior to placebo. In addition, 29% of the CHANTIX group were continuously abstinent from one week after TQD through the end of treatment as compared to 12% of the placebo group and 23% of the bupropion SR group.
Similarly in Study 5, patients treated with CHANTIX had a superior rate of CO-confirmed abstinence during weeks 9 through 12 (44%) compared to patients treated with bupropion SR (30%) or placebo (18%). The bupropion SR quit rate was also superior to placebo. In addition, 29% of the CHANTIX group were continuously abstinent from one week after TQD through the end of treatment as compared to 11% of the placebo group and 21% of the bupropion SR group.
Figure 1: Continuous Abstinence, Weeks 9 through 12
| CHANTIX 0.5 mg BID |
CHANTIX 1 mg BID |
CHANTIX Flexible |
Bupropion SR |
Placebo | |
| BID = twice daily | |||||
| Study 2 | 45% (39%, 51%) |
51% (44%, 57%) |
12% (6%, 18%) |
||
| Study 3 | 40% (32%, 48%) |
12% (7%, 17%) |
|||
| Study 4 | 44% (38%, 49%) |
30% (25%, 35%) |
17% (13%, 22%) |
||
| Study 5 | 44% (38%, 49%) |
30% (25%, 35%) |
18% (14%, 22%) |
||
14.2 Urge to Smoke
Based on responses to the Brief Questionnaire of Smoking Urges and the Minnesota Nicotine Withdrawal scale "urge to smoke" item, CHANTIX reduced urge to smoke compared to placebo in all studies.
14.3 Long-Term Abstinence
Studies 1 through 5 included 40 weeks of post-treatment follow-up. In each study, CHANTIX-treated patients were more likely to maintain abstinence throughout the follow-up period than were patients treated with placebo (Figure 2, Table 3).
Figure 2: Continuous Abstinence, Weeks 9 through 52

| CHANTIX 0.5 mg BID |
CHANTIX 1 mg BID |
CHANTIX Flexible |
Bupropion SR |
Placebo | |
| BID = twice daily | |||||
| Study 2 | 19% (14%, 24%) |
23% (18%, 28%) |
4% (1%, 8%) |
||
| Study 3 | 22% (16%, 29%) |
8% (3%, 12%) |
|||
| Study 4 | 21% (17%, 26%) |
16% (12%, 20%) |
8% (5%, 11%) |
||
| Study 5 | 22% (17%, 26%) |
14% (11%, 18%) |
10% (7%, 13%) |
||
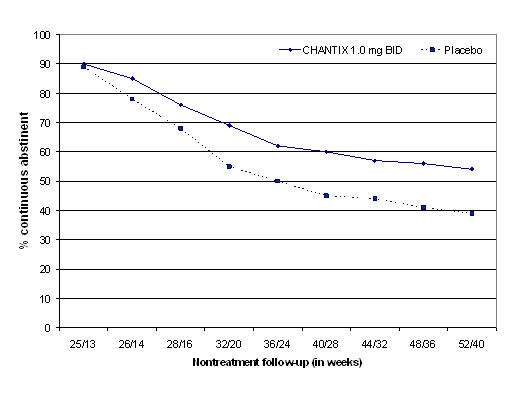
Study 6 : This study assessed the effect of an additional 12 weeks of CHANTIX therapy on the likelihood of long-term abstinence. Patients in this study (n=1927) were treated with open-label CHANTIX 1 mg twice daily for 12 weeks. Patients who had stopped smoking for at least a week by Week 12 (n=1210) were then randomized to double-blind treatment with CHANTIX (1 mg twice daily) or placebo for an additional 12 weeks and then followed for 28 weeks post-treatment.
The continuous abstinence rate from Week 13 through Week 24 was higher for patients continuing treatment with CHANTIX (70%) than for patients switching to placebo (50%). Superiority to placebo was also maintained during 28 weeks post-treatment follow-up (CHANTIX 54% versus placebo 39%).
In Figure 3 below, the x-axis represents the study week for each observation, allowing a comparison of groups at similar times after discontinuation of CHANTIX; post-CHANTIX follow-up begins at Week 13 for the placebo group and Week 25 for the CHANTIX group. The y-axis represents the percentage of patients who had been abstinent for the last week of CHANTIX treatment and remained abstinent at the given timepoint.
Figure 3: Continuous Abstinence Rate during Nontreatment Follow-Up
16 HOW SUPPLIED/STORAGE AND HANDLING
CHANTIX is supplied for oral administration in two strengths: a 0.5 mg capsular biconvex, white to off-white, film-coated tablet debossed with "Pfizer" on one side and "CHX 0.5" on the other side and a 1 mg capsular biconvex, light blue film-coated tablet debossed with "Pfizer" on one side and "CHX 1.0" on the other side. CHANTIX is supplied in the following package configurations:
| Description | NDC | |
| Bottles | 0.5 mg - bottle of 56 | NDC 21695-633-56 |
Store at 25ºC (77ºF); excursions permitted to 15–30ºC (59–86ºF) (see USP Controlled Room Temperature).
17 PATIENT COUNSELING INFORMATION
See Medication Guide
17.1 Set Quit Date and Continue to Attempt to Quit if Lapse
Instruct patients to set a date to quit smoking and to initiate CHANTIX treatment one week before the quit date. Encourage patients to continue to attempt to quit if they have early lapses after quit day [see Dosage and Administration (2.1)].
17.2 How To Take
Advise patients that CHANTIX should be taken after eating, and with a full glass of water [see Dosage and Administration (2.1)].
17.3 Starting Week Dosage
Instruct patients on how to titrate CHANTIX, beginning at a dose of 0.5 mg/day. Explain that one 0.5 mg tablet should be taken daily for the first three days, and that for the next four days, one 0.5 mg tablet should be taken in the morning and one 0.5 mg tablet should be taken in the evening [see Dosage and Administration (2.1)].
17.4 Continuing Weeks Dosage
Advise patients that, after the first seven days, the dose should be increased to one 1 mg tablet in the morning and one 1 mg tablet in the evening [see Dosage and Administration (2.1)].
17.5 Dosage Adjustment for CHANTIX or Other Drugs
Inform patients that nausea and insomnia are side effects of CHANTIX and are usually transient; however, advise patients that if they are persistently troubled by these symptoms, they should notify the prescribing physician so that a dose reduction can be considered.
Inform patients that some drugs may require dose adjustment after quitting smoking [see Dosage and Administration (2.1)].
17.6 Counseling and Support
Provide patients with educational materials and necessary counseling to support an attempt at quitting smoking [see Dosage and Administration (2.1)].
17.7 Neuropsychiatric Symptoms
Inform patients that some patients have experienced changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, anxiety, and panic, as well as suicidal ideation and suicide when attempting to quit smoking while taking CHANTIX. If patients develop agitation, hostility, depressed mood, or changes in behavior or thinking that are not typical for them, or if patients develop suicidal ideation or behavior, they should be urged to discontinue CHANTIX and report these symptoms to their healthcare provider immediately [see Boxed Warning, Warnings and Precautions (5.1), Adverse Reactions (6.2)].
17.8 History of Psychiatric Illness
Encourage patients to reveal any history of psychiatric illness prior to initiating treatment.
17.9 Nicotine Withdrawal
Inform patients that quitting smoking, with or without CHANTIX, may be associated with nicotine withdrawal symptoms (including depression or agitation) or exacerbation of pre-existing psychiatric illness.
17.10 Angioedema
Inform patients that there have been reports of angioedema, with swelling of the face, mouth (lip, gum, tongue) and neck (larynx and pharynx) that can lead to life-threatening respiratory compromise. Instruct patients to discontinue CHANTIX and immediately seek medical care if they experience these symptoms [see Warnings and Precautions (5.2), and Adverse Reactions (6.2)].
17.11 Serious Skin Reactions
Inform patients that serious skin reactions, such as Stevens-Johnson Syndrome and erythema multiforme, were reported by some patients taking CHANTIX. Advise patients to stop taking CHANTIX at the first sign of rash with mucosal lesions or skin reaction and contact a healthcare provider immediately [see Warnings and Precautions (5.3), and Adverse Reactions (6.2)].
17.12 Driving or Operating Machinery
Advise patients to use caution driving or operating machinery until they know how quitting smoking and/or varenicline may affect them [see Warnings and Precautions (5.5)].
17.13 Vivid, Unusual, or Strange Dreams
Inform patients that they may experience vivid, unusual or strange dreams during treatment with CHANTIX.
17.14 Pregnancy and Lactation
Patients who are pregnant or breastfeeding or planning to become pregnant should be advised of: the risks of smoking to a pregnant mother and her developing baby, the potential risks of CHANTIX use during pregnancy and breastfeeding, and the benefits of smoking cessation with and without CHANTIX [see Use in Specific Populations (8.1 and 8.3)].

LAB-0327-12.0
MEDICATION GUIDE
CHANTIX (CHANT-iks)
(varenicline) Tablets
Read the Medication Guide that comes with CHANTIX before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your condition or treatment.
What is the most important information I should know about CHANTIX?
Some people have had changes in behavior, hostility, agitation, depressed mood, and suicidal thoughts or actions while using CHANTIX to help them quit smoking. Some people had these symptoms when they began taking CHANTIX, and others developed them after several weeks of treatment, or after stopping CHANTIX.
If you, your family, or caregiver notice agitation, hostility, depression or changes in behavior or thinking that are not typical for you, or you develop any of the following symptoms, stop taking CHANTIX and call your healthcare provider right away:
- thoughts about suicide or dying, or attempts to commit suicide
- new or worse depression, anxiety, or panic attacks
- feeling very agitated or restless
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- abnormal thoughts or sensations
- seeing or hearing things that are not there (hallucinations)
- feeling people are against you (paranoia)
- feeling confused
- other unusual changes in behavior or mood
When you try to quit smoking, with or without CHANTIX, you may have symptoms that may be due to nicotine withdrawal, including urge to smoke, depressed mood, trouble sleeping, irritability, frustration, anger, feeling anxious, difficulty concentrating, restlessness, decreased heart rate, and increased appetite or weight gain. Some people have even experienced suicidal thoughts when trying to quit smoking without medication. Sometimes quitting smoking can lead to worsening of mental health problems that you already have, such as depression.
Before taking CHANTIX, tell your doctor if you have ever had depression or other mental health problems. You should also tell your doctor about any symptoms you had during other times you tried to quit smoking, with or without CHANTIX.
See " What are the possible side effects of CHANTIX? "
Some people can have allergic reactions to CHANTIX. Some of these allergic reactions can be life-threatening and include: swelling of the face, mouth, and throat that can cause trouble breathing. If you have these symptoms, stop taking CHANTIX and get medical attention right away.
Some people can have serious skin reactions while taking CHANTIX. These can include rash, swelling, redness, and peeling of the skin. Some of these reactions can become life-threatening. If you have a rash with peeling skin or blisters in your mouth, stop taking CHANTIX and see your doctor right away.
What is CHANTIX?
CHANTIX is a prescription medicine to help adults stop smoking.
Quitting smoking can lower your chances of having lung disease, heart disease or getting certain types of cancer that are related to smoking.
CHANTIX is not recommended for people under 18 years of age.
CHANTIX has not been studied with other treatments for stopping smoking.
Who should not take CHANTIX?
Do not take CHANTIX if you have had a serious allergic or skin reaction to CHANTIX, which may include:
- swelling of the face, mouth, and throat that can cause trouble breathing.
- rash, swelling, redness, and peeling of the skin.
What should I tell my doctor before taking CHANTIX?
Before you take CHANTIX, tell your doctor if you:
- have ever had depression or other mental health problems. See "What is the most important information I should know about CHANTIX?"
- have kidney problems or get kidney dialysis. Your doctor may prescribe a lower dose of CHANTIX for you.
- have any allergies. See the end of this Medication Guide for a complete list of ingredients in CHANTIX.
- have any other medical conditions
- are pregnant or plan to become pregnant. Ask your doctor for help to stop smoking before you get pregnant because smoking during pregnancy puts you and your baby at risk for problems during pregnancy. CHANTIX has not been studied in pregnant women. It is not known if CHANTIX will harm your unborn baby.
- are breastfeeding. CHANTIX has not been studied in breastfeeding women. It is not known if CHANTIX passes into breast milk. You and your doctor should talk about the best way to feed your baby if you take CHANTIX.
Tell your doctor about all your other medicines, including prescription and nonprescription medicines, vitamins and herbal supplements. Especially, tell your doctor if you take:
- insulin
- asthma medicines
- blood thinners
When you stop smoking, there may be a change in how these and other medicines work for you.
You should not use CHANTIX while using other medicines to quit smoking. Tell your doctor if you use other treatments to quit smoking.
Know the medicines you take. Keep a list of them with you to show your doctor and pharmacist when you get a new medicine.
How should I take CHANTIX?
- Take CHANTIX exactly as prescribed by your doctor.
- Choose a quit date when you will stop smoking.
- Start taking CHANTIX 1 week (7 days) before your quit date. This lets CHANTIX build up in your body. You can keep smoking during this time. Make sure that you try and stop smoking on your quit date. If you slip-up and smoke, try again. Some people need to take CHANTIX for a few weeks for CHANTIX to work best.
- Take CHANTIX after eating and with a full glass (8 ounces) of water.
- Most people will take CHANTIX for up to 12 weeks. If you have completely quit smoking by 12 weeks, your doctor may prescribe CHANTIX for another 12 weeks to help you stay cigarette-free.
- CHANTIX comes as a white tablet (0.5 mg) and a blue tablet (1 mg). You start with the white tablet and then usually go to the blue tablet. See the chart below for dosing instructions.
-
Day 1 to Day 3 - White tablet (0.5 mg)
- Take 1 tablet each day
Day 4 to Day 7 - White tablet (0.5 mg)
- Take 1 in the morning and 1 in the evening
Day 8 to end of treatment - Blue tablet (1 mg)
- Take 1 in the morning and 1 in the evening
- This dosing schedule may not be right for everyone. Talk to your doctor if you are having side effects such as nausea, strange dreams, or sleep problems. Your doctor may want to reduce your dose.
- If you miss a dose of CHANTIX, take it as soon as you remember. If it is close to the time for your next dose, wait. Just take your next dose at your regular dose.
What should I avoid while taking CHANTIX?
Use caution driving or operating machinery until you know how CHANTIX may affect you. Some people who use CHANTIX may feel sleepy, dizzy, or have trouble concentrating, that can make it hard to drive or perform other activities safely.
What are the possible side effects of CHANTIX?
- Some patients have had new or worse mental health problems. See "What is the most important information I should know about CHANTIX?"
- The most common side effects of CHANTIX include:
- nausea
- sleep problems (trouble sleeping or vivid, unusual, or strange dreams)
- constipation
- gas
- vomiting
Tell your doctor about side effects that bother you or that do not go away.
These are not all the side effects of CHANTIX. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store CHANTIX?
- Store CHANTIX at room temperature, 59 to 86°F (15 to 30°C).
- Safely dispose of CHANTIX that is out of date or no longer needed.
- Keep CHANTIX and all medicines out of the reach of children.
General information about CHANTIX
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use CHANTIX for a condition for which it was not prescribed. Do not give your CHANTIX to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about CHANTIX. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about CHANTIX that is written for healthcare professionals.
For more about CHANTIX and tips on how to quit smoking, go to www.CHANTIX.com or call 1-877-CHANTIX (877-242-6849).
What are the ingredients in CHANTIX?
Active ingredient: varenicline tartrate
Inactive ingredients: microcrystalline cellulose, anhydrous dibasic calcium phosphate, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, Opadry ® White (for 0.5 mg), Opadry ® Blue (for 1 mg), and Opadry® Clear (for both 0.5 mg and 1 mg)
Rx only

LAB-0328-9.0
April 2010
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
This Medication Guide has been approved by the U.S. Food and Drug Administration.
PRINCIPAL DISPLAY PANEL - 0.5 mg Label
Rx only
56 Tablets
NDC 21695-633-56
Chantix™
(varenicline)
tablets
0.5 mg*
Pfizer
Distributed by
Pfizer Labs
Division of Pfizer Inc, NY, NY 10017
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320

Chantixvarenicline tartrate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||