Childrens Sabadil
Consumer label Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Allium cepa 5C, Ambrosia artemisiaefolia 5C, Histaminum hydrochloricum 9C, Euphrasia officinalis 5C, Sabadilla 5C, Solidago virgaurea 5C.
Uses
For temporary relief of itchy nose, sneezing, runny nose, and itchy and watery eyes associated with hay fever or other upper respiratory allergies.
Do not use if label sealing clear cap is broken.
Children 2 years of age and older: At the onset of symptoms, take 5 pellets every 15 minutes for 1 hour. Then take 5 pellets 3 times a day. Allow the pellets to disolve in the mouth. Children younger than 2 years of age: Ask a doctor.
lactose, sucrose.
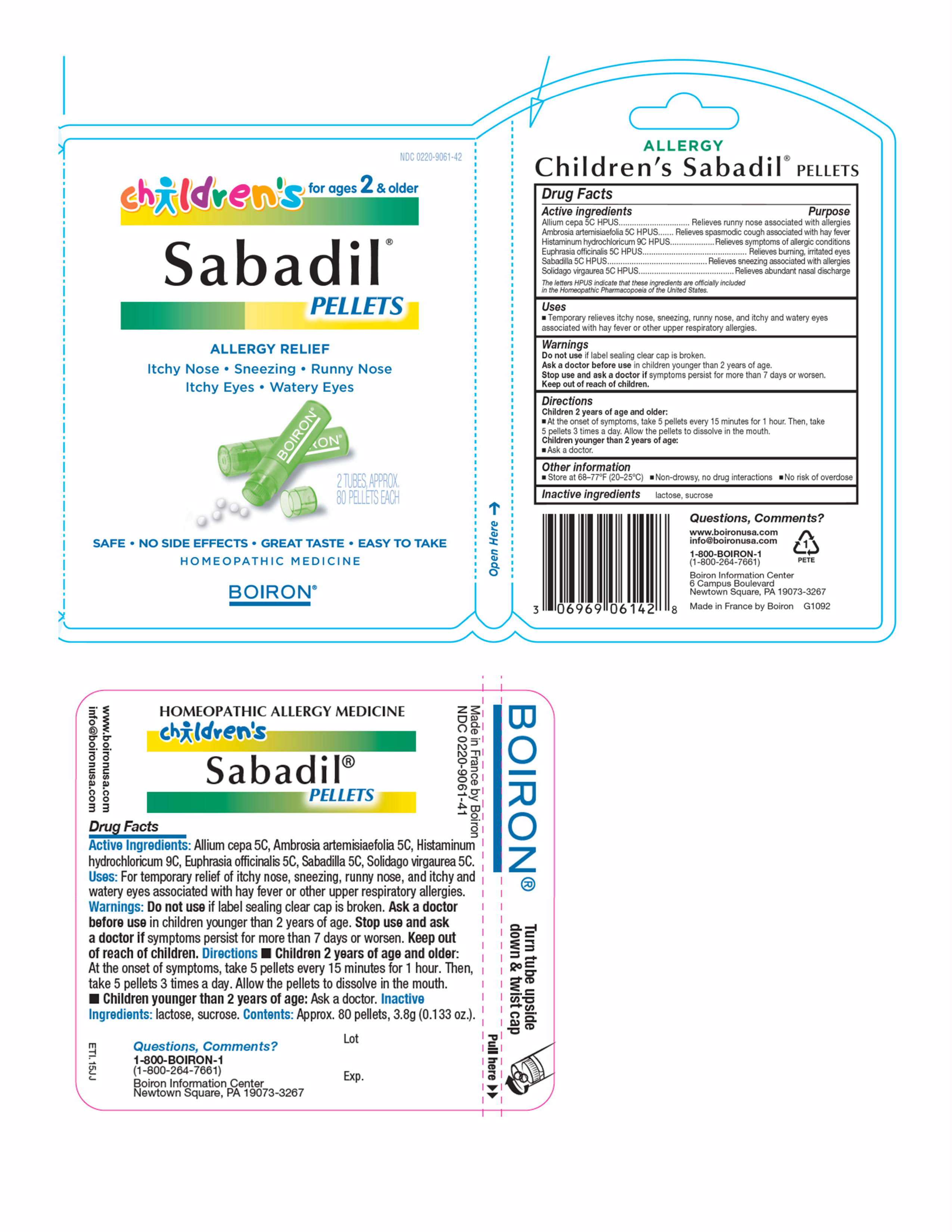
Approx. 80 pellets, 3.8 g (0.133 oz.).
Purpose
N/A
Questions, Comments?
1-800-BOIRON-1
(1-800-264-7661)
Boiron Information Center
Ask doctor before use in children younger than 2 years of age.
Stop use and ask doctor if symptoms persist for more than 7 days or worsen.
N/A
N/A
Childrens SabadilONION, AMBROSIA ARTEMISIIFOLIA, HISTAMINE DIHYDROCHLORIDE, SCHOENOCAULON OFFICINALE SEED, EUPHRASIA STRICTA, SOLIDAGO VIRGAUREA FLOWERING TOP PELLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||