Ciprofloxacin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- CIPROFLOXACIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- DRUG INTERACTIONS
- USE IN SPECIAL POPULATIONS
- MICROBIOLOGY
- CIPROFLOXACIN HYDROCHLORIDE INDICATIONS AND USAGE
- PEDIATRIC USE
- CIPROFLOXACIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CIPROFLOXACIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- CIPROFLOXACIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- ANIMAL PHARMACOLOGY
- CLINICAL STUDIES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
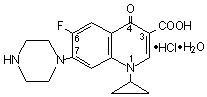
CIPROFLOXACIN HYDROCHLORIDE DESCRIPTION
1718332
CLINICAL PHARMACOLOGY
|
Dose (mg) |
Maximum Serum Concentrations (mcg/mL) |
Area Under Curve (AUC) (mcg•hr/mL) |
|---|---|---|
|
250 500 750 1000 |
1.2 2.4 4.3 5.4 |
4.8 11.6 20.2 30.8 |
max
|
Steady-state Pharmacokinetic Parameters
Following Multiple Oral and I.V. Doses |
||||
|---|---|---|---|---|
|
Parameters AUC (mcg•hr/mL) Cmax (mcg/mL) |
500 mg q12h, P.O. 13.7a 2.97 |
400 mg q12h, I.V. 12.7a 4.56 |
750 mg q12h, P.O. 31.6b 3.59 |
400 mg q8h, I.V. 32.9c 4.07 |
b0-12h
c0-8h
DRUG INTERACTIONS
USE IN SPECIAL POPULATIONS
Pharmacokinetic studies of the oral (single dose) and intravenous (single and
multiple dose) forms of ciprofloxacin indicate that plasma concentrations of
ciprofloxacin are higher in elderly subjects (> 65 years) as compared to
young adults. Although the Cmax is increased 16 - 40%,
the increase in mean AUC is approximately 30%, and can be at least partially
attributed to decreased renal clearance in the elderly. Elimination half-life is
only slightly (~20%) prolonged in the elderly. These differences are not
considered clinically significant. (See
PRECAUTIONS: Geriatric
Use
.)
MICROBIOLOGY
Ciprofloxacin has in vitro activity against a wide
range of gram-negative and gram-positive microorganisms. The bactericidal action
of ciprofloxacin results from inhibition of the enzymes topoisomerase II (DNA
gyrase) and topoisomerase IV, which are required for bacterial DNA replication,
transcription, repair, and recombination. The mechanism of action of
fluoroquinolones, including ciprofloxacin, is different from that of
penicillins, cephalosporins, aminoglycosides, macrolides, and tetracyclines;
therefore, microorganisms resistant to these classes of drugs may be susceptible
to ciprofloxacin and other quinolones. There is no known cross-resistance
between ciprofloxacin and other classes of antimicrobials. In vitro resistance to ciprofloxacin develops slowly by
multiple step mutations. Ciprofloxacin is slightly less active when tested at
acidic pH. The inoculum size has little effect when tested in vitro. The minimal bactericidal concentration (MBC)
generally does not exceed the minimal inhibitory concentration (MIC) by more
than a factor of 2.
Ciprofloxacin has been shown to be active against
most strains of the following microorganisms, both
in vitro
and in clinical infections as described in the
INDICATIONS AND
USAGE
section of the package outsert for Ciprofloxacin Tablets.
1
a
| MIC (μg/mL) | Interpretation |
|---|---|
|
≤ 1 |
Susceptible (S) |
|
2 |
Intermediate (I) |
|
≥ 4 |
Resistant
(R) |
a
b
| MIC (μg/mL) | Interpretation |
|---|---|
|
≤ 1 |
Susceptible
(S) |
b 1
c
| MIC (μg/mL) | Interpretation |
|---|---|
|
≤ 0.06 |
Susceptible (S) |
|
0.12 – 0.5 |
Intermediate (I) |
|
≥ 1 |
Resistant
(R) |
c
| Organism | MIC (μg/mL) | |
|---|---|---|
|
E. faecalis
|
ATCC 29212 |
0.25 – 2 |
|
E. coli
|
ATCC 25922 |
0.004 – 0.015 |
|
H.
influenzae
a
|
ATCC 49247 |
0.004 – 0.03 |
|
P. aeruginosa
|
ATCC 27853 |
0.25 – 1 |
|
S. aureus
|
ATCC 29213 |
0.12 – 0.5 |
|
C. jejuni
b
|
ATCC 33560 |
0.06 – 0.25 and 0.03 – 0.12 |
|
N.
gonorrhoeae
c
|
ATCC 49226 |
0.001 –
0.008 |
a 1
b oo2
c 2o33
a
| Zone Diameter (mm) | Interpretation |
|---|---|
|
≥ 21 |
Susceptible (S) |
|
16 – 20 |
Intermediate (I) |
|
≤ 15 |
Resistant
(R) |
a 2
b
| Zone Diameter (mm) | Interpretation |
|---|---|
|
≥ 21 |
Susceptible
(S) |
b 3
c
| Zone Diameter (mm) | Interpretation |
|---|---|
|
≥41 |
Susceptible (S) |
|
28 – 40 |
Intermediate (I) |
|
≤ 27 |
Resistant
(R) |
c
| Organism |
|
Zone Diameter (mm) |
|---|---|---|
|
E. coli
|
ATCC 25922 |
30 – 40 |
|
H.
influenzae
a
|
ATCC 49247 |
34 – 42 |
|
N.
gonorrhoeae
b
|
ATCC 49226 |
48 – 58 |
|
P. aeruginosa
|
ATCC 27853 |
25 – 33 |
|
S. aureus
|
ATCC 25923 |
22 –
30 |
a 3
b
CIPROFLOXACIN HYDROCHLORIDE INDICATIONS AND USAGE
, Shigella dysenteriae, Shigella flexneri or Shigella sonnei
PEDIATRIC USE
5
CIPROFLOXACIN HYDROCHLORIDE CONTRAINDICATIONS
Ciprofloxacin is contraindicated in persons with a history of hypersensitivity
to ciprofloxacin, any member of the quinolone class of antimicrobial agents, or
any of the product components.
Concomitant administration with
tizanidine is contraindicated. (See
PRECAUTIONS: Drug
Interactions
.)
WARNINGS
Fluoroquinolones, including Ciprofloxacin Tablets, are associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and rupture of the Achilles tendon may require surgical repair. Tendinitis and tendon rupture in the rotater cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites have also been reported. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Factors, in addition to age and corticosteroid use, that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors. Tendon rupture can occur during or after completion of therapy; cases occurring up to several months after completion of therapy have been reported. Ciprofloxacin Tablets should be discontinued if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
- fever, rash, or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome);
- vasculitis; arthralgia; myalgia; serum sickness;
- allergic pneumonitis;
- interstitial nephritis; acute renal insufficiency or failure;
- hepatitis; jaundice; acute hepatic necrosis or failure;
- anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
The drug should be discontinued immediately at the first appearance of a skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted (See PRECAUTIONS: Information for Patients and ADVERSE REACTIONS ).
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Ciprofloxacin Tablets, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile .
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
PRECAUTIONS
- to contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue Ciprofloxacin Tablets treatment. The risk of severe tendon disorder with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- that antibacterial drugs including Ciprofloxacin Tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Ciprofloxacin Tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Ciprofloxacin Tablets or other antibacterial drugs in the future.
- that ciprofloxacin may be taken with or without meals and to drink fluids liberally. As with other quinolones, concurrent administration of ciprofloxacin with magnesium/aluminum antacids, or sucralfate, Videx® (didanosine) chewable/buffered tablets or pediatric powder, other highly buffered drugs, or with other products containing calcium, iron or zinc should be avoided. Ciprofloxacin may be taken two hours before or six hours after taking these products. Ciprofloxacin should not be taken with dairy products (like milk or yogurt) or calcium-fortified juices alone since absorption of ciprofloxacin may be significantly reduced; however, ciprofloxacin may be taken with a meal that contains these products.
- that ciprofloxacin may be associated with hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash or other allergic reaction.
- that photosensitivity/phototoxicity has been reported in patients receiving quinolones. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking quinolones. If patients need to be outdoors while using quinolones, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, patients should contact their physician.
- that peripheral neuropathies have been associated with ciprofloxacin use. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, they should discontinue treatment and contact their physicians.
- that ciprofloxacin may cause dizziness and lightheadedness; therefore, patients should know how they react to this drug before they operate an automobile or machinery or engage in activities requiring mental alertness or coordination.
- that ciprofloxacin increases the effects of tizanidine (Zanaflex®). Patients should not use ciprofloxacin if they are already taking tizanidine.
- that ciprofloxacin may increase the effects of theophylline and caffeine. There is a possibility of caffeine accumulation when products containing caffeine are consumed while taking quinolones.
- that convulsions have been reported in patients receiving quinolones, including ciprofloxacin, and to notify their physician before taking this drug if there is a history of this condition.
- that ciprofloxacin has been associated with an increased rate of adverse events involving joints and surrounding tissue structures (like tendons) in pediatric patients (less than 18 years of age). Parents should inform their child’s physician if the child has a history of joint-related problems before taking this drug. Parents of pediatric patients should also notify their child’s physician of any joint-related problems that occur during or following ciprofloxacin therapy. (See WARNINGS, PRECAUTIONS, Pediatric Use and ADVERSE REACTIONS .)
- that diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
DRUG INTERACTIONS
In a pharmacokinetic study, systemic exposure of tizanidine (4 mg single dose)
was significantly increased (Cmax 7-fold, AUC 10-fold)
when the drug was given concomitantly with ciprofloxacin (500 mg bid for 3
days). The hypotensive and sedative effects of tizanidine were also potentiated.
Concomitant administration of tizanidine and ciprofloxacin is
contraindicated.
As with some other quinolones, concurrent administration
of ciprofloxacin with theophylline may lead to elevated serum concentrations of
theophylline and prolongation of its elimination half-life. This may result in
increased risk of theophylline-related adverse reactions. (See
WARNINGS
.) If
concomitant use cannot be avoided, serum levels of theophylline should be
monitored and dosage adjustments made as appropriate.
Some quinolones,
including ciprofloxacin, have also been shown to interfere with the metabolism
of caffeine. This may lead to reduced clearance of caffeine and a prolongation
of its serum half-life.
Concurrent administration of a quinolone,
including ciprofloxacin, with multivalent cation-containing products such as
magnesium/aluminum antacids, sucralfate,Videx®
(didanosine) chewable/buffered tablets or pediatric powder, other highly
buffered drugs, or products containing calcium, iron, or zinc may substantially
decrease its absorption, resulting in serum and urine levels considerably lower
than desired. (See
DOSAGE AND
ADMINISTRATION
for concurrent administration of these agents with
ciprofloxacin.)
Histamine H2-receptor antagonists
appear to have no significant effect on the bioavailability of
ciprofloxacin.
Altered serum levels of phenytoin (increased and
decreased) have been reported in patients receiving concomitant
ciprofloxacin.
The concomitant administration of ciprofloxacin with the
sulfonylurea glyburide has, on rare occasions, resulted in severe
hypoglycemia.
Some quinolones, including ciprofloxacin, have been
associated with transient elevations in serum creatinine in patients receiving
cyclosporine concomitantly.
Quinolones, including ciprofloxacin, have
been reported to enhance the effects of the oral anticoagulant warfarin or its
derivatives. When these products are administered concomitantly, prothrombin
time or other suitable coagulation tests should be closely
monitored.
Probenecid interferes with renal tubular secretion of
ciprofloxacin and produces an increase in the level of ciprofloxacin in the
serum. This should be considered if patients are receiving both drugs
concomitantly.
Renal tubular transport of methotrexate may be inhibited
by concomitant administration of ciprofloxacin potentially leading to increased
plasma levels of methotrexate. This might increase the risk of methotrexate
associated toxic reactions. Therefore, patients under methotrexate therapy
should be carefully monitored when concomitant ciprofloxacin therapy is
indicated.
Metoclopramide significantly accelerates the absorption of
oral ciprofloxacin resulting in shorter time to reach maximum plasma
concentrations. No significant effect was observed on the bioavailability of
ciprofloxacin.
Non-steroidal anti-inflammatory drugs (but not acetyl
salicylic acid) in combination of very high doses of quinolones have been shown
to provoke convulsions in pre-clinical studies.
79
2
24
2
PREGNANCY
8
9
10
8,9
22
NURSING MOTHERS
Ciprofloxacin is excreted in human milk. The amount of ciprofloxacin absorbed by the nursing infant is unknown. Because of the potential for serious adverse reactions in infants nursing from mothers taking ciprofloxacin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
PEDIATRIC USE
Ciprofloxacin, like other quinolones, causes arthropathy and histological changes in weight-bearing joints of juvenile animals resulting in lameness. (See ANIMAL PHARMACOLOGY .)
Ciprofloxacin is indicated for the treatment of complicated urinary tract infections and pyelonephritis due to Escherichia coli. Although effective in clinical trials, ciprofloxacin is not a drug of first choice in the pediatric population due to an increased incidence of adverse events compared to the controls, including events related to joints and/or surrounding tissues. The rates of these events in pediatric patients with complicated urinary tract infection and pyelonephritis within six weeks of follow-up were 9.3% (31/335) versus 6% (21/349) for control agents. The rates of these events occurring at any time up to the one year follow-up were 13.7% (46/335) and 9.5% (33/349), respectively. The rate of all adverse events regardless of drug relationship at six weeks was 41% (138/335) in the ciprofloxacin arm compared to 31% (109/349) in the control arm. (See ADVERSE REACTIONS . )
Short-term safety data from a single trial in pediatric cystic fibrosis patients are available. In a randomized, double-blind clinical trial for the treatment of acute pulmonary exacerbations in cystic fibrosis patients (ages 5 - 17 years), 67 patients received ciprofloxacin I.V. 10 mg/kg/dose q8h for one week followed by ciprofloxacin tablets 20 mg/kg/dose q12h to complete 10 - 21 days treatment and 62 patients received the combination of ceftazidime I.V. 50 mg/kg/dose q8h and tobramycin I.V. 3 mg/kg/dose q8h for a total of 10 - 21 days. Patients less than 5 years of age were not studied. Safety monitoring in the study included periodic range of motion examinations and gait assessments by treatment-blinded examiners. Patients were followed for an average of 23 days after completing treatment (range 0 - 93 days). This study was not designed to determine long term effects and the safety of repeated exposure to ciprofloxacin.
Musculoskeletal adverse events in patients with cystic fibrosis were reported in 22% of the patients in the ciprofloxacin group and 21% in the comparison group. Decreased range of motion was reported in 12% of the subjects in the ciprofloxacin group and 16% in the comparison group. Arthralgia was reported in 10% of the patients in the ciprofloxacin group and 11% in the comparison group. Other adverse events were similar in nature and frequency between treatment arms. One of sixty-seven patients developed arthritis of the knee nine days after a ten-day course of treatment with ciprofloxacin. Clinical symptoms resolved, but an MRI showed knee effusion without other abnormalities eight months after treatment. However, the relationship of this event to the patient’s course of ciprofloxacin can not be definitively determined, particularly since patients with cystic fibrosis may develop arthralgias/arthritis as part of their underlying disease process.
GERIATRIC USE
Geriatric patients are at increased risk for developing severe tendon disorders
including tendon rupture when being treated with a fluoroquinolone such as
Ciprofloxacin Tablets. This risk is further increased in patients receiving
concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the
Achilles, hand, shoulder, or other tendon sites and can occur during or after
completion of therapy; cases occurring up to several months after
fluoroquinolone treatment have been reported. Caution should be used when
prescribing Ciprofloxacin Tablets to elderly patients especially those on
corticosteroids. Patients should be informed of this potential side effect and
advised to discontinue Ciprofloxacin Tablets and contact their healthcare
provider if any symptoms of tendinitis or tendon rupture occur (See
Boxed Warning
, WARNINGS,
and
ADVERSE
REACTIONS/Post-Marketing Adverse Event Reports
).
In a
retrospective analysis of 23 multiple-dose controlled clinical trials of
ciprofloxacin encompassing over 3500 ciprofloxacin treated patients, 25% of
patients were greater than or equal to 65 years of age and 10% were greater than
or equal to 75 years of age. No overall differences in safety or effectiveness
were observed between these subjects and younger subjects, and other reported
clinical experience has not identified differences in responses between the
elderly and younger patients, but greater sensitivity of some older individuals
on any drug therapy cannot be ruled out. Ciprofloxacin is known to be
substantially excreted by the kidney, and the risk of adverse reactions may be
greater in patients with impaired renal function. No alteration of dosage is
necessary for patients greater than 65 years of age with normal renal function.
However, since some older individuals experience reduced renal function by
virtue of their advanced age, care should be taken in dose selection for elderly
patients, and renal function monitoring may be useful in these patients. (See
CLINICAL
PHARMACOLOGY
and
DOSAGE AND
ADMINISTRATION
.)
In general, elderly patients may be more
susceptible to drug-associated effects on the QT interval. Therefore, precaution
should be taken when using Ciprofloxacin Tablets with concomitant drugs that can
result in prolongation of the QT interval (e.g., class IA or class III
antiarrhythmics) or in patients with risk factors for torsade de pointes (e.g.,
known QT prolongation, uncorrected hypokalemia).
CIPROFLOXACIN HYDROCHLORIDE ADVERSE REACTIONS
Ciprofloxacin, administered I.V. and/or orally, was compared to a
cephalosporin for treatment of complicated urinary tract infections (cUTI) or
pyelonephritis in pediatric patients 1 to 17 years of age (mean age of 6 ± 4
years). The trial was conducted in the U.S., Canada, Argentina, Peru, Costa
Rica, Mexico, South Africa, and Germany. The duration of therapy was 10 to 21
days (mean duration of treatment was 11 days with a range of 1 to 88 days). The
primary objective of the study was to assess musculoskeletal and neurological
safety within 6 weeks of therapy and through one year of follow-up in the 335
ciprofloxacin- and 349 comparator-treated patients enrolled.
An
Independent Pediatric Safety Committee (IPSC) reviewed all cases of
musculoskeletal adverse events as well as all patients with an abnormal gait or
abnormal joint exam (baseline or treatment-emergent). These events were
evaluated in a comprehensive fashion and included such conditions as arthralgia,
abnormal gait, abnormal joint exam, joint sprains, leg pain, back pain,
arthrosis, bone pain, pain, myalgia, arm pain, and decreased range of motion in
a joint.
The affected joints included: knee, elbow, ankle, hip, wrist, and shoulder. Within 6 weeks of treatment initiation, the rates of these events were 9.3% (31/335) in the ciprofloxacin-treated group versus 6% (21/349) in comparator-treated patients. The majority of these events were mild or moderate in intensity. All musculoskeletal events occurring by 6 weeks resolved (clinical resolution of signs and symptoms), usually within 30 days of end of treatment.
Radiological evaluations were not routinely used to confirm resolution of the
events. The events occurred more frequently in ciprofloxacin-treated patients
than control patients, regardless of whether they received I.V. or oral therapy.
Ciprofloxacin-treated patients were more likely to report more than one event
and on more than one occasion compared to control patients. These events
occurred in all age groups and the rates were consistently higher in the
ciprofloxacin group compared to the control group. At the end of 1 year, the
rate of these events reported at any time during that period was 13.7% (46/335)
in the ciprofloxacin-treated group versus 9.5% (33/349) comparator-treated
patients.
An adolescent female discontinued ciprofloxacin for wrist pain
that developed during treatment. An MRI performed 4 weeks later showed a tear in
the right ulnar fibrocartilage. A diagnosis of overuse syndrome secondary to
sports activity was made, but a contribution from ciprofloxacin cannot be
excluded. The patient recovered by 4 months without surgical
intervention.
| Ciprofloxacin | Comparator | |
|---|---|---|
|
*The study was designed to demonstrate that the arthropathy rate for the
ciprofloxacin group did not exceed that of the control group by more than + 6%.
At both the 6 week and 1 year evaluations, the 95% confidence interval indicated
that it could not be concluded that ciprofloxacin group had findings comparable
to the control group. |
||
|
All Patients (within 6 weeks)
|
31/335 (9.3%) |
21/349 (6%) |
|
95% Confidence Interval* |
(-0.8%, +7.2%) |
|
|
Age Group |
|
|
|
less then 12 months greater then 24 months
|
1/36 (2.8%) |
0/41 |
|
less then 2 years greater then 6 years |
5/124 (4%) |
3/118 (2.5%) |
|
less then 6 years greater then 12 years |
18/143 (12.6%) |
12/153 (7.8%) |
|
less then 12 years to 17 years |
7/32 (21.9%) |
6/37 (16.2 %) |
|
All Patients (within 1 year)
|
46/335 (13.7%) |
33/349 (9.5%) |
|
95% Confidence Interval* |
(-0.6%, +
9.1%) |
|
Changes in laboratory parameters listed as adverse events without
regard to drug relationship are listed below:
Hepatic – Elevations of ALT (SGPT) (1.9%), AST (SGOT) (1.7%), alkaline phosphatase (0.8%),
LDH (0.4%), serum bilirubin (0.3%).
Hematologic – Eosinophilia
(0.6%), leukopenia (0.4%), decreased blood platelets (0.1%), elevated blood
platelets (0.1%), pancytopenia (0.1%).
Renal – Elevations
of serum creatinine (1.1%), BUN (0.9%), CRYSTALLURIA, CYLINDRURIA, AND
HEMATURIA HAVE BEEN REPORTED.
Other changes occurring in less than 0.1%
of courses were: elevation of serum gammaglutamyl transferase, elevation of
serum amylase, reduction in blood glucose, elevated uric acid, decrease in
hemoglobin, anemia, bleeding diathesis, increase in blood monocytes,
leukocytosis.
To report SUSPECTED ADVERSE EVENTS, contact West-ward Pharmaceutical Corp. at 1-877-233-2001 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
In the event of acute overdosage, reversible renal toxicity has been reported in
some cases. The stomach should be emptied by inducing vomiting or by gastric
lavage. The patient should be carefully observed and given supportive treatment,
including monitoring of renal function and administration of magnesium,
aluminum, or calcium containing antacids which can reduce the absorption of
ciprofloxacin. Adequate hydration must be maintained. Only a small amount of
ciprofloxacin (greater then 10%) is removed from the body after hemodialysis or
peritoneal dialysis.
Single doses of ciprofloxacin were relatively
non-toxic via the oral route of administration in mice, rats, and dogs. No
deaths occurred within a 14-day post treatment observation period at the highest
oral doses tested; up to 5000 mg/kg in either rodent species, or up to 2500
mg/kg in the dog. Clinical signs observed included hypoactivity and cyanosis in
both rodent species and severe vomiting in dogs. In rabbits, significant
mortality was seen at doses of ciprofloxacin less then 2500 mg/kg. Mortality was
delayed in these animals, occurring 10 to 14 days after dosing.
In mice,
rats, rabbits and dogs, significant toxicity including tonic/clonic convulsions
was observed at intravenous doses of ciprofloxacin between 125 and 300 mg/kg.
CIPROFLOXACIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
| ADULT DOSAGE GUIDELINES | ||||
|---|---|---|---|---|
| Infection | Severity | Dose | Frequency | Usual Durations |
|
Urinary Tract |
Acute Uncomplicated |
250 mg |
q 12 h |
3 Days |
|
Mild/Moderate |
250 mg |
q 12 h |
7 to 14 Days |
|
|
Severe/Complicated |
500 mg |
q 12 h |
7 to 14 Days |
|
|
Chronic Bacterial Prostatitis |
Mild/Moderate |
500 mg |
q 12 h |
28 Days |
|
Lower Respiratory Tract |
Mild/Moderate |
500 mg |
q 12 h |
7 to 14 days |
|
Severe/Complicated |
750 mg |
q 12 h |
7 to 14 days |
|
|
Acute Sinusitis |
Mild/Moderate |
500 mg |
q 12 h |
10 days |
|
Skin and Skin Structure |
Mild/Moderate |
500 mg |
q 12 h |
7 to 14 Days |
|
Severe/Complicated |
750 mg |
q 12 h |
7 to 14 Days |
|
|
Bone and Joint |
Mild/Moderate |
500 mg |
q 12 h |
≥4 to 6 weeks |
|
Severe/Complicated |
750 mg |
q 12 h |
≥4 to 6 weeks |
|
|
Intra-Abdominal*
|
Complicated |
500 mg |
q 12 h |
7 to 14 Days |
|
Infectious Diarrhea |
Mild/Moderate/Severe |
500 mg |
q 12 h |
5 to 7 Days |
|
Typhoid Fever |
Mild/Moderate |
500 mg |
q 12 h |
10 Days |
|
Urethral and Cervical Gonococcal Infections |
Uncomplicated |
250 mg |
single dose |
single dose |
|
Inhalational anthrax(post-exposure)**
|
|
500 mg |
q 12 h |
60 Days |
used in
conjunction with metronidazole
Generally ciprofloxacin should be continued for at least 2 days
after the signs and symptoms of infection
have disappeared, except for
inhalational anthrax (post-exposure).
Drug
administration should begin as soon as possible after suspected or confirmed
exposure.
This indication is based on a surrogate endpoint,
ciprofloxacin serum concentrations achieved in humans,
reasonably likely
to predict clinical benefit.4 For a discussion of
ciprofloxacin serum concentrations in various
human populations, see
INHALATIONAL ANTHRAX –
ADDITIONAL INFORMATION
.
Conversion of I.V. to
Oral Dosing in Adults
Patients whose therapy is started with
ciprofloxacin I.V. may be switched to Ciprofloxacin Tablets when clinically
indicated at the discretion of the physician (See
CLINICAL
PHARMACOLOGY
and table below for the equivalent dosing
regimens).
| Ciprofloxacin Oral Dosage | Equivalent Ciprofloxacin I.V. Dosage |
|---|---|
|
250 mg Tablet q 12 h |
200 mg I.V. q 12 h |
|
500 mg Tablet q 12 h |
400 mg I.V. q 12 h |
|
750 mg Tablet q 12 h |
400 mg I.V. q 8
h |
| Creatinine Clearance (mL/min) | Dose |
|---|---|
|
less then 50 |
See Usual Dosage. |
|
30 – 50 |
250 – 500 mg q 12 h |
|
5 – 29 |
250 – 500 mg q 18 h |
|
Patients on hemodialysis or Peritoneal dialysis |
250 – 500 mg q 24 h (after dialysis) |
| Infection |
Route of Administration |
Dose (mg/kg) |
Frequency |
Total Duration |
|---|---|---|---|---|
|
Complicated Urinary Tract or Pyelonephritis |
Intravenous |
6 to 10 mg/kg (maximum 400 mg per dose; not to be exceeded even in patients weighing > 51 kg) |
Every 8 hours |
10-21 days* |
|
(patients from 1 to 17 years of age) |
Oral |
10 mg/kg to 20 mg/kg (maximum 750 mg per dose; not to be exceeded even in patients weighing > 51 kg) |
Every 12 hours |
|
|
Inhalational Anthrax (Post-Exposure)** |
Intravenous |
10 mg/kg (maximum 400 mg per dose) |
Every 12 hours |
60 days |
|
Oral |
15 mg/kg (maximum 500 mg per dose) |
Every 12
hours |
5
2
HOW SUPPLIED
Ciprofloxacin Tablets USP, are available as white, round, film-coated tablets containing 250 mg ciprofloxacin. The 250 mg tablet is coded with "WW927" on one side.
Ciprofloxacin Tablets, USP area also available as white, capsule shaped, film-coated tablets containing 500 mg or 750 mg ciprofloxacin. The 500 mg tablet is coded with "WW928" on one side. The 750 mg tablet is coded with "WW929" on one side. Ciprofloxacin Tablets, USP 250 mg and 500 mg are available in bottles of 30's, 100's and 500's.
Ciprofloxacin Tablets, USP 750 mg are available in bottles of 50's and 100's and Unit Dose Boxes of 100 tablets.
Strength Tablet Identification
Bottles of 30's: 250
mg WW927
500
mg WW928
Bottles of
50's: 750
mg WW929
Bottles of
100's: 250
mg WW927
500
mg WW928
750
mg WW929
Bottles of
500's: 250
mg WW927
500
mg WW928
Unit Dose Boxes of
100 250
mg WW927
500
mg WW928
750
mg WW929
Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature]. Dispense in a well-closed container as defined in the USP using a child-resistant closure.
ANIMAL PHARMACOLOGY
22
CLINICAL STUDIES
|
|
Ciprofloxacin | Comparator |
|---|---|---|
|
* Patients with baseline pathogen(s) eradicated and no new infections
or superinfections/total number of patients. There were 5.5% (6/211) ciprofloxacin and 9.5% (22/231) comparator patients with superinfections or new infections. |
||
|
Randomized Patients |
337 |
352 |
|
Per Protocol Patients |
211 |
231 |
|
Clinical Response at 5 to 9
Days Post-Treatment |
95.7% (202/211) |
92.6% (214/231) |
|
|
95% CI [-1.3%,
7.3%] |
|
|
Bacteriologic Eradication by Patient at 5 to 9 Days Post-Treatment* |
84.4% (178/211) |
78.3% (181/231) |
|
|
95% CI [-1.3%,
13.1%] |
|
|
Bacteriologic Eradication of
the Baseline Pathogen at 5 to 9 Days Post-Treatment |
|
|
|
Escherichia coli
|
156/178 (88%) |
161/179
(90%) |
The mean serum concentrations of ciprofloxacin associated with a statistically significant improvement in survival in the rhesus monkey model of inhalational anthrax are reached or exceeded in adult and pediatric patients receiving oral and intravenous regimens. (See DOSAGE AND ADMINISTRATION .) Ciprofloxacin pharmacokinetics have been evaluated in various human populations. The mean peak serum concentration achieved at steady-state in human adults receiving 500 mg orally every 12 hours is 2.97 μg/mL, and 4.56 μg/mL following 400 mg intravenously every 12 hours. The mean trough serum concentration at steady-state for both of these regimens is 0.2 μg/mL.
In a study of 10 pediatric patients between 6 and 16 years of age, the mean
peak plasma concentration achieved is 8.3 μg/mL and trough concentrations range
from 0.09 to 0.26 μg/mL, following two 30-minute intravenous infusions of 10
mg/kg administered 12 hours apart. After the second intravenous infusion
patients switched to 15 mg/kg orally every 12 hours achieve a mean peak
concentration of 3.6 μg/mL after the initial oral dose. Long-term safety data,
including effects on cartilage, following the administration of ciprofloxacin to
pediatric patients are limited. (For additional information, see
PRECAUTIONS,
Pediatric Use
.) Ciprofloxacin serum concentrations achieved in humans
serve as a surrogate endpoint reasonably likely to predict clinical benefit and
provide the basis for this indication.5
A
placebo-controlled animal study in rhesus monkeys exposed to an inhaled mean
dose of 11 LD50 (~5.5 x 105 spores
(range 5 to 30 LD50) of
B. anthracis
was conducted. The minimal inhibitory concentration (MIC) of
ciprofloxacin for the anthrax strain used in this study was 0.08 μg/mL. In the
animals studied, mean serum concentrations of ciprofloxacin achieved at expected
Tmax (1 hour post-dose) following oral dosing to
steady-state ranged from 0.98 to 1.69 μg/mL. Mean steady-state trough
concentrations at 12 hours post-dose ranged from 0.12 to 0.19 μg/mL.6 Mortality due to anthrax for animals that received a 30-day
regimen of oral ciprofloxacin beginning 24 hours post-exposure was significantly
lower (1/9), compared to the placebo group (9/10) [p=0.001]. The one
ciprofloxacin-treated animal that died of anthrax did so following the 30-day
drug administration period.7
More than 9300
persons were recommended to complete a minimum of 60 days of antibiotic
prophylaxis against possible inhalational exposure to
B.
anthracis
during 2001. Ciprofloxacin was recommended to most of those
individuals for all or part of the prophylaxis regimen. Some persons were also
given anthrax vaccine or were switched to alternative antibiotics. No one who
received ciprofloxacin or other therapies as prophylactic treatment subsequently
developed inhalational anthrax. The number of persons who received ciprofloxacin
as all or part of their post-exposure prophylaxis regimen is
unknown.
Among the persons surveyed by the Centers for Disease Control
and Prevention, over 1000 reported receiving ciprofloxacin as sole post-exposure
prophylaxis for inhalational anthrax. Gastrointestinal adverse events (nausea,
vomiting, diarrhea, or stomach pain), neurological adverse events (problems
sleeping, nightmares, headache, dizziness or lightheadedness) and
musculoskeletal adverse events (muscle or tendon pain and joint swelling or
pain) were more frequent than had been previously reported in controlled
clinical trials. This higher incidence, in the absence of a control group, could
be explained by a reporting bias, concurrent medical conditions, other
concomitant medications, emotional stress or other confounding factors, and/or a
longer treatment period with ciprofloxacin. Because of these factors and
limitations in the data collection, it is difficult to evaluate whether the
reported symptoms were drug-related.
- National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically -Fifth Edition. Approved Standard NCCLS Document M7-A5, Vol. 20, No. 2, NCCLS, Wayne, PA, January, 2000.
- Clinical and Laboratory Standards Institute, Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guideline. , CLSI Document M45-A, Vol. 26, No. 19, CLSI, Wayne, PA, 2006.
- National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disk Susceptibility Tests -Seventh Edition. Approved Standard NCCLS Document M2-A7, Vol. 20, No. 1, NCCLS, Wayne, PA, January, 2000.
- Report presented at the FDA’s Anti-Infective Drug and Dermatological Drug Product’s Advisory Committee meeting, March 31, 1993, Silver Spring, MD. Report available from FDA, CDER, Advisors and Consultants Staff, HFD-21, 1901 Chapman Avenue, Room 200, Rockville, MD20852, USA.
- 21 CFR 314.510 (Subpart H – Accelerated Approval of New Drugs for Life-Threatening Illnesses).
- Kelly DJ, et al. Serum concentrations of penicillin, doxycycline, and ciprofloxacin during prolonged therapy in rhesus monkeys. J Infect Dis 1992; 166:1184-7.
- Friedlander AM, et al. Postexposure prophylaxis against experimental inhalational anthrax. J Infect Dis 1993; 167:1239-42.
- Friedman J, Polifka J. Teratogenic effects of drugs: a resource for clinicians (TERIS). Baltimore, Maryland: Johns Hopkins University Press, 2000:149-195.
- Loebstein R, Addis A, Ho E, et al. Pregnancy outcome following gestational exposure to fluoroquinolones: a multicenter prospective controlled study. Antimicrob Agents Chemother. 1998;42(6):1336-1339.
- Schaefer C, Amoura-Elefant E, Vial T, et al. Pregnancy outcome after prenatal quinolone exposure. Evaluation of a case registry of the European network of teratology information services (ENTIS). Eur J Obstet Gynecol Reprod Biol. 1996;69:83-89.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


Ciprofloxacin HydrochlorideCiprofloxacin Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||