Clarinex
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLARINEX safely and effectively. See full prescribing information for CLARINEX. CLARINEX (desloratadine) Tablets, RediTabs, and Oral Solution for oral useInitial U.S. Approval: 2001RECENT MAJOR CHANGES Dosage and Administration (2) 04/2014 Contraindications (4) 04/2014 INDICATIONS AND USAGE CLARINEX is an H1-receptor antagonist indicated for: Seasonal Allergic Rhinitis: relief of nasal and non-nasal symptoms in patients 2 years of age and older. (1.1) Perennial Allergic Rhinitis: relief of nasal and non-nasal symptoms in patients 6 months of age and older. (1.2) Chronic Idiopathic Urticaria: symptomatic relief of pruritus, reduction in the number of hives, and size of hives in patients 6 months of age and older. (1.3) DOSAGE AND ADMINISTRATION Dosage (by age): Adults and Adolescents 12 Years of Age and Over: CLARINEX Tablets - one 5 mg tablet once daily or CLARINEX RediTabs Tablets - one 5 mg tablet once daily or CLARINEX Oral Solution - 2 teaspoonfuls (5 mg in 10 mL) once daily (2) Children 6 to 11 Years of Age: CLARINEX Oral Solution - 1 teaspoonful (2.5 mg in 5 mL) once daily or CLARINEX RediTabs Tablets - one 2.5 mg tablet once daily (2) Children 12 Months to 5 Years of Age: CLARINEX Oral Solution - 1/2 teaspoonful (1.25 mg in 2.5 mL) once daily (2) Children 6 to 11 Months of Age: CLARINEX Oral Solution - 2 mL (1 mg) once daily (2) DOSAGE FORMS AND STRENGTHS CLARINEX Tablets - 5 mg (3) CLARINEX Oral Solution - 0.5 mg/1 mL (3) CONTRAINDICATIONS Hypersensitivity (4, 6.2) WARNINGS AND PRECAUTIONS Hypersensitivity reactions including rash, pruritus, urticaria, edema, dyspnea, and anaphylaxis have been reported. In such cases, stop CLARINEX at once and consider alternative treatments. (5.1) Side Effects The most common adverse reactions (reported in ≥2% of adult and adolescent patients with allergic rhinitis and greater than placebo) were pharyngitis, dry mouth, myalgia, fatigue, somnolence, dysmenorrhea. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONS Renal impairment: dosage adjustment is recommended (2.5, 8.6, 12.3) Hepatic impairment: dosage adjustment is recommended (2.5, 8.7, 12.3)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 CLARINEX INDICATIONS AND USAGE
- 2 CLARINEX DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CLARINEX CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 CLARINEX ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 CLARINEX DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Seasonal Allergic Rhinitis
CLARINEX® is indicated for the relief of the nasal and non-nasal symptoms of seasonal allergic rhinitis in patients 2 years of age and older.
1.2 Perennial Allergic Rhinitis
CLARINEX is indicated for the relief of the nasal and non-nasal symptoms of perennial allergic rhinitis in patients 6 months of age and older.
1.3 Chronic Idiopathic Urticaria
CLARINEX is indicated for the symptomatic relief of pruritus, reduction in the number of hives, and size of hives, in patients with chronic idiopathic urticaria 6 months of age and older.
2 DOSAGE AND ADMINISTRATION
Although an orally disintegrating tablet formulation of desloratadine may be available in the marketplace, CLARINEX® RediTabs® Tablets are no longer marketed.
CLARINEX Tablets, Oral Solution, or RediTabs Tablets may be taken without regard to meals. Place CLARINEX (desloratadine) RediTabs Tablets on the tongue and allow to disintegrate before swallowing. Tablet disintegration occurs rapidly. Administer with or without water. Take tablet immediately after opening the blister.
The age-appropriate dose of CLARINEX Oral Solution should be administered with a commercially available measuring dropper or syringe that is calibrated to deliver 2 mL and 2.5 mL (½ teaspoon).
2.1 Adults and Adolescents 12 Years of Age and Over
The recommended dose of CLARINEX Tablets or CLARINEX RediTabs Tablets is one 5-mg tablet once daily. The recommended dose of CLARINEX Oral Solution is 2 teaspoonfuls (5 mg in 10 mL) once daily.
2.2 Children 6 to 11 Years of Age
The recommended dose of CLARINEX Oral Solution is 1 teaspoonful (2.5 mg in 5 mL) once daily. The recommended dose of CLARINEX RediTabs Tablets is one 2.5-mg tablet once daily.
2.3 Children 12 Months to 5 Years of Age
The recommended dose of CLARINEX Oral Solution is ½ teaspoonful (1.25 mg in 2.5 mL) once daily.
2.4 Children 6 to 11 Months of Age
The recommended dose of CLARINEX Oral Solution is 2 mL (1 mg) once daily.
2.5 Adults with Hepatic or Renal Impairment
In adult patients with liver or renal impairment, a starting dose of one 5-mg tablet every other day is recommended based on pharmacokinetic data. Dosing recommendation for children with liver or renal impairment cannot be made due to lack of data [see Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
CLARINEX Tablets are light blue, film-coated tablets embossed with "C5" containing 5 mg desloratadine.
CLARINEX Oral Solution is a clear orange-colored liquid containing 0.5 mg desloratadine/1 mL.
4 CONTRAINDICATIONS
CLARINEX Tablets, RediTabs, and Oral Solution are contraindicated in patients who are hypersensitive to this medication or to any of its ingredients or to loratadine [see Warnings and Precautions (5.1) and Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions including rash, pruritus, urticaria, edema, dyspnea, and anaphylaxis have been reported after administration of desloratadine. If such a reaction occurs, therapy with CLARINEX should be stopped and alternative treatment should be considered. [See Adverse Reactions (6.2).]
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Hypersensitivity reactions. [See Warnings and Precautions (5.1).]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adults and Adolescents
Allergic Rhinitis: In multiple-dose placebo-controlled trials, 2834 patients ages 12 years or older received CLARINEX Tablets at doses of 2.5 mg to 20 mg daily, of whom 1655 patients received the recommended daily dose of 5 mg. In patients receiving 5 mg daily, the rate of adverse events was similar between CLARINEX and placebo-treated patients. The percent of patients who withdrew prematurely due to adverse events was 2.4% in the CLARINEX group and 2.6% in the placebo group. There were no serious adverse events in these trials in patients receiving desloratadine. All adverse events that were reported by greater than or equal to 2% of patients who received the recommended daily dose of CLARINEX Tablets (5 mg once daily), and that were more common with CLARINEX Tablets than placebo, are listed in Table 1.
| Adverse Event | CLARINEX Tablets 5 mg (n=1655) |
Placebo (n=1652) |
|---|---|---|
| Infections and Infestations | ||
| Pharyngitis | 4.1% | 2.0% |
| Nervous System Disorders | ||
| Somnolence | 2.1% | 1.8% |
| Gastrointestinal Disorders | ||
| Dry Mouth | 3.0% | 1.9% |
| Musculoskeletal and Connective Tissue Disorders | ||
| Myalgia | 2.1% | 1.8% |
| Reproductive System and Breast Disorders | ||
| Dysmenorrhea | 2.1% | 1.6% |
| General Disorders and Administration Site Conditions | ||
| Fatigue | 2.1% | 1.2% |
The frequency and magnitude of laboratory and electrocardiographic abnormalities were similar in CLARINEX and placebo-treated patients.
There were no differences in adverse events for subgroups of patients as defined by gender, age, or race.
Chronic Idiopathic Urticaria: In multiple-dose, placebo-controlled trials of chronic idiopathic urticaria, 211 patients ages 12 years or older received CLARINEX Tablets and 205 received placebo. Adverse events that were reported by greater than or equal to 2% of patients who received CLARINEX Tablets and that were more common with CLARINEX than placebo were (rates for CLARINEX and placebo, respectively): headache (14%, 13%), nausea (5%, 2%), fatigue (5%, 1%), dizziness (4%, 3%), pharyngitis (3%, 2%), dyspepsia (3%, 1%), and myalgia (3%, 1%).
Pediatrics
Two hundred and forty-six pediatric subjects 6 months to 11 years of age received CLARINEX Oral Solution for 15 days in three placebo-controlled clinical trials. Pediatric subjects aged 6 to 11 years received 2.5 mg once a day, subjects aged 1 to 5 years received 1.25 mg once a day, and subjects 6 to 11 months of age received 1.0 mg once a day.
In subjects 6 to 11 years of age, no individual adverse event was reported by 2 percent or more of the subjects.
In subjects 2 to 5 years of age, adverse events reported for CLARINEX and placebo in at least 2 percent of subjects receiving CLARINEX Oral Solution and at a frequency greater than placebo were fever (5.5%, 5.4%), urinary tract infection (3.6%, 0%) and varicella (3.6%, 0%).
In subjects 12 months to 23 months of age, adverse events reported for the CLARINEX product and placebo in at least 2 percent of subjects receiving CLARINEX Oral Solution and at a frequency greater than placebo were fever (16.9%, 12.9%), diarrhea (15.4%, 11.3%), upper respiratory tract infections (10.8%, 9.7%), coughing (10.8%, 6.5%), appetite increased (3.1%, 1.6%), emotional lability (3.1%, 0%), epistaxis (3.1%, 0%), parasitic infection (3.1%, 0%), pharyngitis (3.1%, 0%), rash maculopapular (3.1%, 0%).
In subjects 6 months to 11 months of age, adverse events reported for CLARINEX and placebo in at least 2 percent of subjects receiving CLARINEX Oral Solution and at a frequency greater than placebo were upper respiratory tract infections (21.2%, 12.9%), diarrhea (19.7%, 8.1%), fever (12.1%, 1.6%), irritability (12.1%, 11.3%), coughing (10.6%, 9.7%), somnolence (9.1%, 8.1%), bronchitis (6.1%, 0%), otitis media (6.1%, 1.6%), vomiting (6.1%, 3.2%), anorexia (4.5%, 1.6%), pharyngitis (4.5%, 1.6%), insomnia (4.5%, 0%), rhinorrhea (4.5%, 3.2%), erythema (3.0%, 1.6%), and nausea (3.0%, 0%).
There were no clinically meaningful changes in any electrocardiographic parameter, including the QTc interval. Only one of the 246 pediatric subjects receiving CLARINEX Oral Solution in the clinical trials discontinued treatment because of an adverse event.
6.2 Post-Marketing Experience
Because adverse events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following spontaneous adverse events have been reported during the marketing of desloratadine: tachycardia, palpitations, rare cases of hypersensitivity reactions (such as rash, pruritus, urticaria, edema, dyspnea, and anaphylaxis), psychomotor hyperactivity, movement disorders (including dystonia, tics, and extrapyramidal symptoms), seizures, and elevated liver enzymes including bilirubin, and very rarely, hepatitis.
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P450 3A4
In controlled clinical studies co-administration of desloratadine with ketoconazole, erythromycin, or azithromycin resulted in increased plasma concentrations of desloratadine and 3 hydroxydesloratadine, but there were no clinically relevant changes in the safety profile of desloratadine. [See Clinical Pharmacology (12.3).]
7.2 Fluoxetine
In controlled clinical studies co-administration of desloratadine with fluoxetine, a selective serotonin reuptake inhibitor (SSRI), resulted in increased plasma concentrations of desloratadine and 3 hydroxydesloratadine, but there were no clinically relevant changes in the safety profile of desloratadine. [See Clinical Pharmacology (12.3).]
7.3 Cimetidine
In controlled clinical studies co-administration of desloratadine with cimetidine, a histamine H2-receptor antagonist, resulted in increased plasma concentrations of desloratadine and 3 hydroxydesloratadine, but there were no clinically relevant changes in the safety profile of desloratadine. [See Clinical Pharmacology (12.3).]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, desloratadine should be used during pregnancy only if clearly needed.
Desloratadine was not teratogenic in rats or rabbits at approximately 210 and 230 times, respectively, the area under the concentration-time curve (AUC) in humans at the recommended daily oral dose. An increase in pre-implantation loss and a decreased number of implantations and fetuses were noted, however, in a separate study in female rats at approximately 120 times the AUC in humans at the recommended daily oral dose. Reduced body weight and slow righting reflex were reported in pups at approximately 50 times or greater than the AUC in humans at the recommended daily oral dose. Desloratadine had no effect on pup development at approximately 7 times the AUC in humans at the recommended daily oral dose. The AUCs in comparison referred to the desloratadine exposure in rabbits and the sum of desloratadine and its metabolites exposures in rats, respectively. [See Nonclinical Toxicology (13.2).]
8.3 Nursing Mothers
Desloratadine passes into breast milk; therefore, a decision should be made whether to discontinue nursing or to discontinue desloratadine, taking into account the benefit of the drug to the nursing mother and the possible risk to the child.
8.4 Pediatric Use
The recommended dose of CLARINEX Oral Solution in the pediatric population is based on cross-study comparison of the plasma concentration of CLARINEX in adults and pediatric subjects. The safety of CLARINEX Oral Solution has been established in 246 pediatric subjects aged 6 months to 11 years in three placebo-controlled clinical studies. Since the course of seasonal and perennial allergic rhinitis and chronic idiopathic urticaria and the effects of CLARINEX are sufficiently similar in the pediatric and adult populations, it allows extrapolation from the adult efficacy data to pediatric patients. The effectiveness of CLARINEX Oral Solution in these age groups is supported by evidence from adequate and well-controlled studies of CLARINEX Tablets in adults. The safety and effectiveness of CLARINEX Tablets or CLARINEX Oral Solution have not been demonstrated in pediatric patients less than 6 months of age. [See Clinical Pharmacology (12.3).]
The CLARINEX RediTabs 2.5-mg tablet has not been evaluated in pediatric patients. Bioequivalence of the CLARINEX RediTabs Tablet and the previously marketed RediTabs Tablet was established in adults. In conjunction with the dose-finding studies in pediatrics described, the pharmacokinetic data for CLARINEX RediTabs supports the use of the 2.5-mg dose strength in pediatric patients 6 to 11 years of age.
8.5 Geriatric Use
Clinical studies of desloratadine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. [See Clinical Pharmacology (12.3).]
8.6 Renal Impairment
Dosage adjustment for patients with renal impairment is recommended [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Dosage adjustment for patients with hepatic impairment is recommended [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
There is no information to indicate that abuse or dependency occurs with CLARINEX Tablets.
10 OVERDOSAGE
In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Desloratadine and 3-hydroxydesloratadine are not eliminated by hemodialysis.
Information regarding acute overdosage is limited to experience from post-marketing adverse event reports and from clinical trials conducted during the development of the CLARINEX product. In a dose-ranging trial, at doses of 10 mg and 20 mg/day somnolence was reported.
In another study, no clinically relevant adverse events were reported in normal male and female volunteers who were given single daily doses of CLARINEX 45 mg for 10 days [see Clinical Pharmacology (12.2)].
Lethality occurred in rats at oral doses of 250 mg/kg or greater (estimated desloratadine and desloratadine metabolite exposures were approximately 120 times the AUC in humans at the recommended daily oral dose). The oral median lethal dose in mice was 353 mg/kg (estimated desloratadine exposures were approximately 290 times the human daily oral dose on a mg/m2 basis). No deaths occurred at oral doses up to 250 mg/kg in monkeys (estimated desloratadine exposures were approximately 810 times the human daily oral dose on a mg/m2 basis).
11 DESCRIPTION
CLARINEX (desloratadine) Tablets are light blue, round, film-coated tablets containing 5 mg desloratadine, an antihistamine, to be administered orally. CLARINEX Tablets also contain the following excipients: dibasic calcium phosphate dihydrate USP, microcrystalline cellulose NF, corn starch NF, talc USP, carnauba wax NF, white wax NF, coating material consisting of lactose monohydrate, hypromellose, titanium dioxide, polyethylene glycol, and FD&C Blue #2 Aluminum Lake.
CLARINEX Oral Solution is a clear orange-colored liquid containing 0.5 mg/1 mL desloratadine. The Oral Solution contains the following inactive ingredients: propylene glycol USP, sorbitol solution USP, citric acid (anhydrous) USP, sodium citrate dihydrate USP, sodium benzoate NF, disodium edetate USP, purified water USP. It also contains granulated sugar, natural and artificial flavor for bubble gum, and FDC Yellow #6 dye.
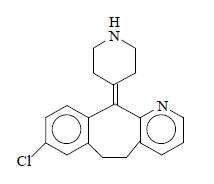
Desloratadine is a white to off-white powder that is slightly soluble in water, but very soluble in ethanol and propylene glycol. It has an empirical formula: C19H19ClN2 and a molecular weight of 310.8. The chemical name is 8-chloro-6,11-dihydro-11-(4-piperdinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine and has the following structure:
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Desloratadine is a long-acting tricyclic histamine antagonist with selective H1-receptor histamine antagonist activity. Receptor binding data indicates that at a concentration of 2–3 ng/mL (7 nanomolar), desloratadine shows significant interaction with the human histamine H1-receptor. Desloratadine inhibited histamine release from human mast cells in vitro. Results of a radiolabeled tissue distribution study in rats and a radioligand H1-receptor binding study in guinea pigs showed that desloratadine did not readily cross the blood brain barrier. The clinical significance of this finding is unknown.
12.2 Pharmacodynamics
Wheal and Flare: Human histamine skin wheal studies following single and repeated 5-mg doses of desloratadine have shown that the drug exhibits an antihistaminic effect by 1 hour; this activity may persist for as long as 24 hours. There was no evidence of histamine-induced skin wheal tachyphylaxis within the desloratadine 5-mg group over the 28-day treatment period. The clinical relevance of histamine wheal skin testing is unknown.
Effects on QTc: Single daily doses of 45 mg were given to normal male and female volunteers for 10 days. All ECGs obtained in this study were manually read in a blinded fashion by a cardiologist. In CLARINEX-treated subjects, there was an increase in mean heart rate of 9.2 bpm relative to placebo. The QT interval was corrected for heart rate (QTc) by both the Bazett and Fridericia methods. Using the QTc (Bazett) there was a mean increase of 8.1 msec in CLARINEX-treated subjects relative to placebo. Using QTc (Fridericia) there was a mean increase of 0.4 msec in CLARINEX-treated subjects relative to placebo. No clinically relevant adverse events were reported.
12.3 Pharmacokinetics
Absorption
Following oral administration of a desloratadine 5-mg tablet once daily for 10 days to normal healthy volunteers, the mean time to maximum plasma concentrations (Tmax) occurred at approximately 3 hours post dose and mean steady state peak plasma concentrations (Cmax) and AUC of 4 ng/mL and 56.9 ng∙hr/mL were observed, respectively. Neither food nor grapefruit juice had an effect on the bioavailability (Cmax and AUC) of desloratadine.
The pharmacokinetic profile of CLARINEX Oral Solution was evaluated in a three-way crossover study in 30 adult volunteers. A single dose of 10 mL of CLARINEX Oral Solution containing 5 mg of desloratadine was bioequivalent to a single dose of 5-mg CLARINEX Tablet. Food had no effect on the bioavailability (AUC and Cmax) of CLARINEX Oral Solution.
The pharmacokinetic profile of CLARINEX RediTabs Tablets was evaluated in a three-way crossover study in 24 adult volunteers. A single CLARINEX RediTabs Tablet containing 5 mg of desloratadine was bioequivalent to a single 5-mg CLARINEX RediTabs Tablet (original formulation) for both desloratadine and 3-hydroxydesloratadine. Food and water had no effect on the bioavailability (AUC and Cmax) of CLARINEX RediTabs Tablets.
Distribution
Desloratadine and 3-hydroxydesloratadine are approximately 82% to 87% and 85% to 89% bound to plasma proteins, respectively. Protein binding of desloratadine and 3-hydroxydesloratadine was unaltered in subjects with impaired renal function.
Metabolism
Desloratadine (a major metabolite of loratadine) is extensively metabolized to 3-hydroxydesloratadine, an active metabolite, which is subsequently glucuronidated. The enzyme(s) responsible for the formation of 3-hydroxydesloratadine have not been identified. Data from clinical trials indicate that a subset of the general population has a decreased ability to form 3-hydroxydesloratadine, and are poor metabolizers of desloratadine. In pharmacokinetic studies (n=3748), approximately 6% of subjects were poor metabolizers of desloratadine (defined as a subject with an AUC ratio of 3-hydroxydesloratadine to desloratadine less than 0.1, or a subject with a desloratadine half-life exceeding 50 hours). These pharmacokinetic studies included subjects between the ages of 2 and 70 years, including 977 subjects aged 2 to 5 years, 1575 subjects aged 6 to 11 years, and 1196 subjects aged 12 to 70 years. There was no difference in the prevalence of poor metabolizers across age groups. The frequency of poor metabolizers was higher in Blacks (17%, n=988) as compared to Caucasians (2%, n=1,462) and Hispanics (2%, n=1,063). The median exposure (AUC) to desloratadine in the poor metabolizers was approximately 6-fold greater than in the subjects who are not poor metabolizers. Subjects who are poor metabolizers of desloratadine cannot be prospectively identified and will be exposed to higher levels of desloratadine following dosing with the recommended dose of desloratadine. In multidose clinical safety studies, where metabolizer status was identified, a total of 94 poor metabolizers and 123 normal metabolizers were enrolled and treated with CLARINEX Oral Solution for 15–35 days. In these studies, no overall differences in safety were observed between poor metabolizers and normal metabolizers. Although not seen in these studies, an increased risk of exposure-related adverse events in patients who are poor metabolizers cannot be ruled out.
Elimination
The mean plasma elimination half-life of desloratadine was approximately 27 hours. Cmax and AUC values increased in a dose proportional manner following single oral doses between 5 and 20 mg. The degree of accumulation after 14 days of dosing was consistent with the half-life and dosing frequency. A human mass balance study documented a recovery of approximately 87% of the 14C-desloratadine dose, which was equally distributed in urine and feces as metabolic products. Analysis of plasma 3-hydroxydesloratadine showed similar Tmax and half-life values compared to desloratadine.
Special Populations
Geriatric Subjects: In older subjects (≥65 years old; n=17) following multiple-dose administration of CLARINEX Tablets, the mean Cmax and AUC values for desloratadine were 20% greater than in younger subjects (<65 years old). The oral total body clearance (CL/F) when normalized for body weight was similar between the two age groups. The mean plasma elimination half-life of desloratadine was 33.7 hr in subjects ≥65 years old. The pharmacokinetics for 3-hydroxydesloratadine appeared unchanged in older versus younger subjects. These age-related differences are unlikely to be clinically relevant and no dosage adjustment is recommended in elderly subjects.
Pediatric Subjects: In subjects 6 to 11 years old, a single dose of 5 mL of CLARINEX Oral Solution containing 2.5 mg of desloratadine, resulted in desloratadine plasma concentrations similar to those achieved in adults administered a single 5-mg CLARINEX Tablet. In subjects 2 to 5 years old, a single dose of 2.5 mL of CLARINEX Oral Solution containing 1.25 mg of desloratadine, resulted in desloratadine plasma concentrations similar to those achieved in adults administered a single 5-mg CLARINEX Tablet. However, the Cmax and AUC of the metabolite (3-hydroxydesloratadine) were 1.27 and 1.61 times higher for the 5-mg dose of Oral Solution administered in adults compared to the Cmax and AUC obtained in children 2 to 11 years of age receiving 1.25–2.5 mg of CLARINEX Oral Solution.
A single dose of either 2.5 mL or 1.25 mL of CLARINEX Oral Solution containing 1.25 mg or 0.625 mg, respectively, of desloratadine was administered to subjects 6 to 11 months of age and 12 to 23 months of age. The results of a population pharmacokinetic analysis indicated that a dose of 1 mg for subjects aged 6 to 11 months and 1.25 mg for subjects 12 to 23 months of age is required to obtain desloratadine plasma concentrations similar to those achieved in adults administered a single 5-mg dose of CLARINEX Oral Solution.
The CLARINEX RediTabs 2.5-mg tablet has not been evaluated in pediatric patients. Bioequivalence of the CLARINEX RediTabs Tablet and the original CLARINEX RediTabs Tablets was established in adults. In conjunction with the dose-finding studies in pediatrics described, the pharmacokinetic data for CLARINEX RediTabs Tablets supports the use of the 2.5-mg dose strength in pediatric patients 6 to 11 years of age.
Renally Impaired: Desloratadine pharmacokinetics following a single dose of 7.5 mg were characterized in patients with mild (n=7; creatinine clearance 51–69 mL/min/1.73 m2), moderate (n=6; creatinine clearance 34–43 mL/min/1.73 m2), and severe (n=6; creatinine clearance 5–29 mL/min/1.73 m2) renal impairment or hemodialysis dependent (n=6) patients. In patients with mild and moderate renal impairment, median Cmax and AUC values increased by approximately 1.2- and 1.9-fold, respectively, relative to subjects with normal renal function. In patients with severe renal impairment or who were hemodialysis dependent, Cmax and AUC values increased by approximately 1.7- and 2.5-fold, respectively. Minimal changes in 3-hydroxydesloratadine concentrations were observed. Desloratadine and 3-hydroxydesloratadine were poorly removed by hemodialysis. Plasma protein binding of desloratadine and 3-hydroxydesloratadine was unaltered by renal impairment. Dosage adjustment for patients with renal impairment is recommended [see Dosage and Administration (2.5)].
Hepatically Impaired: Desloratadine pharmacokinetics were characterized following a single oral dose in patients with mild (n=4), moderate (n=4), and severe (n=4) hepatic impairment as defined by the Child-Pugh classification of hepatic function and 8 subjects with normal hepatic function. Patients with hepatic impairment, regardless of severity, had approximately a 2.4-fold increase in AUC as compared with normal subjects. The apparent oral clearance of desloratadine in patients with mild, moderate, and severe hepatic impairment was 37%, 36%, and 28% of that in normal subjects, respectively. An increase in the mean elimination half-life of desloratadine in patients with hepatic impairment was observed. For 3-hydroxydesloratadine, the mean Cmax and AUC values for patients with hepatic impairment were not statistically significantly different from subjects with normal hepatic function. Dosage adjustment for patients with hepatic impairment is recommended [see Dosage and Administration (2.5)].
Gender: Female subjects treated for 14 days with CLARINEX Tablets had 10% and 3% higher desloratadine Cmax and AUC values, respectively, compared with male subjects. The 3-hydroxydesloratadine Cmax and AUC values were also increased by 45% and 48%, respectively, in females compared with males. However, these apparent differences are not likely to be clinically relevant and therefore no dosage adjustment is recommended.
Race: Following 14 days of treatment with CLARINEX Tablets, the Cmax and AUC values for desloratadine were 18% and 32% higher, respectively, in Blacks compared with Caucasians. For 3-hydroxydesloratadine there was a corresponding 10% reduction in Cmax and AUC values in Blacks compared to Caucasians. These differences are not likely to be clinically relevant and therefore no dose adjustment is recommended.
Drug Interactions: In two controlled crossover clinical pharmacology studies in healthy male (n=12 in each study) and female (n=12 in each study) volunteers, desloratadine 7.5 mg (1.5 times the daily dose) once daily was coadministered with erythromycin 500 mg every 8 hours or ketoconazole 200 mg every 12 hours for 10 days. In three separate controlled, parallel group clinical pharmacology studies, desloratadine at the clinical dose of 5 mg has been coadministered with azithromycin 500 mg followed by 250 mg once daily for 4 days (n=18) or with fluoxetine 20 mg once daily for 7 days after a 23-day pretreatment period with fluoxetine (n=18) or with cimetidine 600 mg every 12 hours for 14 days (n=18) under steady-state conditions to normal healthy male and female volunteers. Although increased plasma concentrations (Cmax and AUC0-24 hrs) of desloratadine and 3-hydroxydesloratadine were observed (see Table 2), there were no clinically relevant changes in the safety profile of desloratadine, as assessed by electrocardiographic parameters (including the corrected QT interval), clinical laboratory tests, vital signs, and adverse events.
| Desloratadine | 3-Hydroxydesloratadine | |||
|---|---|---|---|---|
| Cmax | AUC0-24 hrs | Cmax | AUC0-24 hrs | |
|
Erythromycin
(500 mg Q8h) |
+ 24% | + 14% | + 43% | + 40% |
| Ketoconazole (200 mg Q12h) |
+ 45% |
+ 39% |
+ 43% |
+ 72% |
| Azithromycin (500 mg day 1, 250 mg QD x 4 days) |
+ 15% |
+ 5% |
+ 15% |
+ 4% |
| Fluoxetine (20 mg QD) |
+ 15% |
+ 0% |
+ 17% |
+ 13% |
| Cimetidine (600 mg Q12h) |
+ 12% |
+ 19% |
- 11% |
- 3% |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity Studies
The carcinogenic potential of desloratadine was assessed using a loratadine study in rats and a desloratadine study in mice. In a 2-year study in rats, loratadine was administered in the diet at doses up to 25 mg/kg/day (estimated desloratadine and desloratadine metabolite exposures were approximately 30 times the AUC in humans at the recommended daily oral dose). A significantly higher incidence of hepatocellular tumors (combined adenomas and carcinomas) was observed in males given 10 mg/kg/day of loratadine and in males and females given 25 mg/kg/day of loratadine. The estimated desloratadine and desloratadine metabolite exposures in rats given 10 mg/kg of loratadine were approximately 7 times the AUC in humans at the recommended daily oral dose. The clinical significance of these findings during long-term use of desloratadine is not known.
In a 2-year dietary study in mice, males and females given up to 16 mg/kg/day and 32 mg/kg/day desloratadine, respectively, did not show significant increases in the incidence of any tumors. The estimated desloratadine and desloratadine metabolite exposures in mice at these doses were 12 and 27 times, respectively, the AUC in humans at the recommended daily oral dose.
Genotoxicity Studies
In genotoxicity studies with desloratadine, there was no evidence of genotoxic potential in a reverse mutation assay (Salmonella/E. coli mammalian microsome bacterial mutagenicity assay) or in 2 assays for chromosomal aberrations (human peripheral blood lymphocyte clastogenicity assay and mouse bone marrow micronucleus assay).
Impairment of Fertility
There was no effect on female fertility in rats at desloratadine doses up to 24 mg/kg/day (estimated desloratadine and desloratadine metabolite exposures were approximately 130 times the AUC in humans at the recommended daily oral dose). A male specific decrease in fertility, demonstrated by reduced female conception rates, decreased sperm numbers and motility, and histopathologic testicular changes, occurred at an oral desloratadine dose of 12 mg/kg in rats (estimated desloratadine and desloratadine metabolite exposures were approximately 45 times the AUC in humans at the recommended daily oral dose). Desloratadine had no effect on fertility in rats at an oral dose of 3 mg/kg/day (estimated desloratadine and desloratadine metabolite exposures were approximately 8 times the AUC in humans at the recommended daily oral dose).
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
Desloratadine was not teratogenic in rats at doses up to 48 mg/kg/day (estimated desloratadine and desloratadine metabolite exposures were approximately 210 times the AUC in humans at the recommended daily oral dose) or in rabbits at doses up to 60 mg/kg/day (estimated desloratadine exposures were approximately 230 times the AUC in humans at the recommended daily oral dose). In a separate study, an increase in pre-implantation loss and a decreased number of implantations and fetuses were noted in female rats at 24 mg/kg (estimated desloratadine and desloratadine metabolite exposures were approximately 120 times the AUC in humans at the recommended daily oral dose). Reduced body weight and slow righting reflex were reported in pups at doses of 9 mg/kg/day or greater (estimated desloratadine and desloratadine metabolite exposures were approximately 50 times or greater than the AUC in humans at the recommended daily oral dose). Desloratadine had no effect on pup development at an oral dose of 3 mg/kg/day (estimated desloratadine and desloratadine metabolite exposures were approximately 7 times the AUC in humans at the recommended daily oral dose).
14 CLINICAL STUDIES
14.1 Seasonal Allergic Rhinitis
The clinical efficacy and safety of CLARINEX Tablets were evaluated in over 2300 patients 12 to 75 years of age with seasonal allergic rhinitis. A total of 1838 patients received 2.5 to 20 mg/day of CLARINEX in 4 double-blind, randomized, placebo-controlled clinical trials of 2 to 4 weeks' duration conducted in the United States. The results of these studies demonstrated the efficacy and safety of CLARINEX 5 mg in the treatment of adult and adolescent patients with seasonal allergic rhinitis. In a dose-ranging trial, CLARINEX 2.5 to 20 mg/day was studied. Doses of 5, 7.5, 10, and 20 mg/day were superior to placebo; and no additional benefit was seen at doses above 5.0 mg. In the same study, an increase in the incidence of somnolence was observed at doses of 10 mg/day and 20 mg/day (5.2% and 7.6%, respectively), compared to placebo (2.3%).
In two 4-week studies of 924 patients (aged 15 to 75 years) with seasonal allergic rhinitis and concomitant asthma, CLARINEX Tablets 5 mg once daily improved rhinitis symptoms, with no decrease in pulmonary function. This supports the safety of administering CLARINEX Tablets to adult patients with seasonal allergic rhinitis with mild to moderate asthma.
CLARINEX Tablets 5 mg once daily significantly reduced the Total Symptom Score (the sum of individual scores of nasal and non-nasal symptoms) in patients with seasonal allergic rhinitis. See Table 3.
| Treatment Group (n) |
Mean Baseline (SEM) |
Change from Baseline (SEM) |
Placebo Comparison (P-value) |
|---|---|---|---|
| SEM=Standard Error of the Mean | |||
|
CLARINEX
5.0 mg (171) |
14.2 (0.3) | -4.3 (0.3) | P<0.01 |
| Placebo (173) | 13.7 (0.3) | -2.5 (0.3) | |
There were no significant differences in the effectiveness of CLARINEX Tablets 5 mg across subgroups of patients defined by gender, age, or race.
14.2 Perennial Allergic Rhinitis
The clinical efficacy and safety of CLARINEX Tablets 5 mg were evaluated in over 1300 patients 12 to 80 years of age with perennial allergic rhinitis. A total of 685 patients received 5 mg/day of CLARINEX in two double-blind, randomized, placebo-controlled clinical trials of 4 weeks' duration conducted in the United States and internationally. In one of these studies CLARINEX Tablets 5 mg once daily was shown to significantly reduce the Total Symptom Score in patients with perennial allergic rhinitis (Table 4).
| Treatment Group (n) |
Mean Baseline (SEM) |
Change from Baseline (SEM) |
Placebo Comparison (P-value) |
|---|---|---|---|
| SEM=Standard Error of the Mean | |||
|
CLARINEX
5.0 mg (337) |
12.37 (0.18) | -4.06 (0.21) | P=0.01 |
| Placebo (337) | 12.30 (0.18) | -3.27 (0.21) | |
14.3 Chronic Idiopathic Urticaria
The efficacy and safety of CLARINEX Tablets 5 mg once daily was studied in 416 chronic idiopathic urticaria patients 12 to 84 years of age, of whom 211 received CLARINEX. In two double-blind, placebo-controlled, randomized clinical trials of six weeks duration, at the pre-specified one-week primary time point evaluation, CLARINEX Tablets significantly reduced the severity of pruritus when compared to placebo (Table 5). Secondary endpoints were also evaluated, and during the first week of therapy CLARINEX Tablets 5 mg reduced the secondary endpoints, "Number of Hives" and the "Size of the Largest Hive," when compared to placebo.
| Treatment Group (n) |
Mean Baseline (SEM) |
Change from Baseline (SEM) |
Placebo Comparison (P-value) |
|---|---|---|---|
| Pruritus scored 0 to 3 where 0=no symptom to 3=maximal symptom | |||
| SEM=Standard Error of the Mean | |||
|
CLARINEX
5.0 mg (115) |
2.19 (0.04) | -1.05 (0.07) | P<0.01 |
| Placebo (110) | 2.21 (0.04) | -0.52 (0.07) | |
The clinical safety of CLARINEX Oral Solution was documented in three, 15-day, double-blind, placebo-controlled safety studies in pediatric subjects with a documented history of allergic rhinitis, chronic idiopathic urticaria, or subjects who were candidates for antihistamine therapy. In the first study, 2.5 mg of CLARINEX Oral Solution was administered to 60 pediatric subjects 6 to 11 years of age. The second study evaluated 1.25 mg of CLARINEX Oral Solution administered to 55 pediatric subjects 2 to 5 years of age. In the third study, 1.25 mg of CLARINEX Oral Solution was administered to 65 pediatric subjects 12 to 23 months of age and 1.0 mg of CLARINEX Oral Solution was administered to 66 pediatric subjects 6 to 11 months of age. The results of these studies demonstrated the safety of CLARINEX Oral Solution in pediatric subjects 6 months to 11 years of age.
16 HOW SUPPLIED/STORAGE AND HANDLING
CLARINEX Tablets: Embossed "C5", light blue, film-coated tablets that are packaged in high-density polyethylene plastic bottles of 100 (NDC 0085-1264-01) and 500 (NDC 0085-1264-02).
CLARINEX Oral Solution: Clear orange-colored liquid containing 0.5 mg/1 mL desloratadine in a 16-ounce Amber glass bottle (NDC 0085-1334-01) and a 4-ounce Amber glass bottle (NDC 0085-1334-02).
Storage
- CLARINEX Tablets: Protect Unit-of-Use packaging and Unit-Dose Hospital Pack from excessive moisture. Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature]. Heat Sensitive. Avoid exposure at or above 30°C (86°F).
- CLARINEX Oral Solution: Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature]. Protect from light.
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information).
17.1 Information for Patients
- Patients should be instructed to use CLARINEX as directed.
- As there are no food effects on bioavailability, patients can be instructed that CLARINEX Tablets, Oral Solution, or RediTabs Tablets may be taken without regard to meals.
- Patients should be advised not to increase the dose or dosing frequency as studies have not demonstrated increased effectiveness at higher doses and somnolence may occur.
- Phenylketonurics: CLARINEX RediTabs Tablets contain phenylalanine.
CLARINEX Tablets and Oral Solution are
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
CLARINEX Tablets are
Manufactured by: Merck Sharp & Dohme Corp., a subsidiary of
Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
CLARINEX Oral Solution is
Manufactured by: Schering-Plough Canada, Inc., Pointe Claire, Quebec, Canada
For patent information: www.merck.com/product/patent/home.html
Copyright © 2004, 2005, 2010 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
uspi-mk4117-mtl-1404r007
PATIENT INFORMATION
CLARINEX® (CLA-RI-NEX) (desloratadine) Tablets, RediTabs®, and Oral Solution
Read the Patient Information that comes with CLARINEX® before you start taking it and each time you get a refill. There may be new information. This leaflet is a summary of the information for patients. Your doctor or pharmacist can give you additional information. This leaflet does not take the place of talking to your doctor about your medical condition or treatment.
What is CLARINEX?
CLARINEX is a prescription medicine that contains the medicine desloratadine (an antihistamine).
CLARINEX is used to help control the symptoms of:
- seasonal allergic rhinitis (sneezing, stuffy nose, runny nose and itching of the nose) in people 2 years of age and older.
- perennial allergic rhinitis (sneezing, stuffy nose, runny nose and itching of the nose) in people 6 months of age and older.
- chronic idiopathic urticaria (long-term itching) and to reduce the number and size of hives in people 6 months of age and older.
CLARINEX is not for children younger than 6 months of age.
Who should not take CLARINEX?
Do not take CLARINEX if you:
- are allergic to desloratadine or any of the ingredients in CLARINEX Tablets, CLARINEX RediTabs® or CLARINEX Oral Solution. See the end of this leaflet for a complete list of ingredients.
- are allergic to loratadine (Alavert, Claritin).
Talk to your doctor before taking this medicine if you have any questions about whether or not to take this medicine.
What should I tell my doctor before taking CLARINEX?
Before you take CLARINEX, tell your doctor if you:
- have liver or kidney problems.
- have any other medical conditions.
- are pregnant or plan to become pregnant. It is not known if CLARINEX will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breast-feeding or plan to breast-feed. CLARINEX can pass into your breast milk. Talk to your doctor about the best way to feed your baby if you take CLARINEX.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. CLARINEX may affect the way other medicines work, and other medicines may affect how CLARINEX works. Especially tell your doctor if you take:
- ketoconazole (Nizoral)
- erythromycin (Ery-tab, Eryc, PCE)
- azithromycin (Zithromax, Zmax)
- antihistamines
- fluoxetine (Prozac)
- cimetidine (Tagamet)
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
How should I take CLARINEX?
- Take CLARINEX exactly as your doctor tells you to take it.
- Do not change your dose of CLARINEX or take more often than prescribed.
- CLARINEX can be taken with or without food.
- Place CLARINEX RediTabs Tablet on your tongue and allow it to dissolve before swallowing. CLARINEX RediTabs can be taken with or without water. Take your CLARINEX RediTabs Tablet right away after opening the blister.
- Take CLARINEX Oral Solution with a measuring dropper or oral syringe that can measure 2 mL or 2.5 mL. Ask your pharmacist for a dropper or syringe if you do not have one.
- If you take too much CLARINEX, call your doctor or get medical attention right away.
What are the possible side effects of CLARINEX Tablets?
CLARINEX may cause serious side effects, including:
- Allergic reactions. Stop taking CLARINEX and call your doctor right away or get emergency help if you have any of these symptoms:
- rash
- itching
- hives
- swelling of your lips, tongue, face, and throat
- shortness of breath or trouble breathing
The most common side effects of CLARINEX in adults and children 12 years of age and older with allergic rhinitis include:
- sore throat
- dry mouth
- muscle pain
- tiredness
- sleepiness
- menstrual pain
Increased sleepiness or tiredness can happen if you take more CLARINEX than your doctor prescribed to you.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of CLARINEX. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store CLARINEX?
- Store CLARINEX Tablets between 59°F to 86°F (15°C to 30°C).
- CLARINEX Tablets are sensitive to heat. Do not store above 86°F (30°C).
- Protect CLARINEX Tablets from moisture.
- Store CLARINEX Oral Solution between 59°F to 86°F (15°C to 30°C). Protect CLARINEX Oral Solution from light.
Keep CLARINEX Tablets, RediTabs Tablets, and Oral Solution and all medicines out of the reach of children.
General information about CLARINEX
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use CLARINEX for a condition for which it was not prescribed. Do not give CLARINEX to other people, even if they have the same condition you have. It may harm them.
This Patient Information leaflet summarizes the most important information about CLARINEX. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about CLARINEX that is written for health professionals.
For more information, go to www.CLARINEX.com
What are the ingredients in CLARINEX?
Active ingredient: desloratadine
Patients with Phenylketonuria: CLARINEX RediTabs Tablets contain phenylalanine.
Inactive ingredients in CLARINEX Tablets: dibasic calcium phosphate dihydrate USP, microcrystalline cellulose NF, corn starch NF, talc USP, carnauba wax NF, white wax NF, coating material consisting of lactose monohydrate, hypromellose, titanium dioxide, polyethylene glycol, and FD&C Blue #2 Aluminum Lake.
Inactive ingredients in CLARINEX Oral Solution: propylene glycol USP, sorbitol solution USP, citric acid (anhydrous) USP, sodium citrate dihydrate USP, sodium benzoate NF, disodium edetate USP, purified water USP. It also contains granulated sugar, natural and artificial flavor for bubble gum and FDC Yellow #6 dye.
CLARINEX Tablets and Oral Solution are
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
CLARINEX Tablets are
Manufactured by: Merck Sharp & Dohme Corp., a subsidiary of
Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
CLARINEX Oral Solution is
Manufactured by: Schering-Plough Canada, Inc., Pointe Claire, Quebec, Canada
For patent information: www.merck.com/product/patent/home.html
The trademarks depicted herein are owned by their respective companies.
Copyright © 2010 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
Revised: 04/2014
usppi-mk4117-mtl-1404r006
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
NDC 0085-1264-01
100 Tablets
CLARINEX
®
(desloratadine)
5 mg TABLETS
Rx only
actual size

PRINCIPAL DISPLAY PANEL - 16 fl oz Bottle Carton
NDC 0085-1334-01
16 fl oz
CLARINEX®
ORAL
SOLUTION
(desloratadine)
0.5 mg per 1 mL
Usual Dose: See package insert.
Each teaspoonful (5 mL) contains:
2.5 mg desloratadine.
Read accompanying directions carefully.
Rx only

ClarinexDesloratadine TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ClarinexDesloratadine SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||