Clindamycin Hydrochloride
Clindamycin Hydrochloride Capsules, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING
- CLINDAMYCIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINDAMYCIN HYDROCHLORIDE INDICATIONS AND USAGE
- CLINDAMYCIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CLINDAMYCIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- CLINDAMYCIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- ANIMAL TOXICOLOGY
- REFERENCES
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg (100 Capsule Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 300 mg (100 Capsule Bottle)
FULL PRESCRIBING INFORMATION
WARNING
Clostridium difficileC. difficile
INDICATIONS AND USAGE
C. difficileC. difficile
C. difficile C. difficile
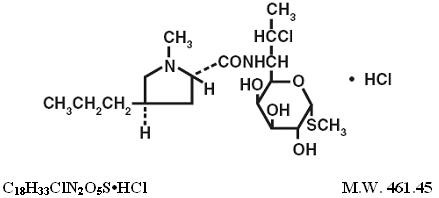
CLINDAMYCIN HYDROCHLORIDE DESCRIPTION
transthreogalacto
CLINICAL PHARMACOLOGY
Human Pharmacology
Special Populations
Microbiology
in vitro
in vitro INDICATIONS AND USAGE
Staphylococcus aureus
Streptococcus pneumoniae
Streptococcus pyogenes
Prevotella melaninogenica
Fusobacterium necrophorum
Fusobacterium nucleatum
Peptostreptococcus anaerobius
Clostridium perfringens
in vitro has not been
Staphylococcus epidermidis
Streptococcus agalactiae
Streptococcus anginosus
Streptococcus oralis
Streptococcus mitis
Prevotella intermedia
Prevotella bivia
Propionibacterium acnes
Micromonas (“Peptostreptococcus”) micros
Finegoldia (“Peptostreptococcus”) magna
Actinomyces israelii
Clostridium clostridioforme
Eubacterium lentum
Susceptibility Testing Methods
in vitro
1,2
1,3
| Pathogen | Susceptibility Interpretive Criteria | |||||
|---|---|---|---|---|---|---|
| Minimal Inhibitory Concentrations (MIC in mcg/mL) |
Disk Diffusion (Zone Diameters in mm) |
|||||
| NA=not applicable |
||||||
|
Staphylococcus spp. |
≤0.5 |
1-2 |
≥4 |
≥21 |
15-20 |
≤14 |
|
Streptococcus pneumoniae and other Streptococcus spp. |
≤0.25 |
0.5 |
≥1 |
≥19 |
16-18 |
≤15 |
| Anaerobic Bacteria
|
≤2 |
4 |
≥8 |
NA |
NA |
NA |
1,2,3,4
| QC Strain | Acceptable Quality Control Ranges | |
|---|---|---|
| Minimum Inhibitory Concentration Range (mcg/mL) |
Disk Diffusion (Zone Diameters in mm) |
|
| NA=not applicable ATCC® is a registered trademark of the American Type Culture Collection |
||
|
When Testing Aerobic Pathogens
|
||
|
Staphylococcus aureus ATCC 29213
|
0.06–0.25
|
NA
|
|
Staphylococcus aureus ATCC 25923
|
NA
|
24–30
|
|
Streptococcus pneumoniae ATCC 49619
|
0.03–0.12
|
19–25
|
|
When Testing Anaerobes
|
||
|
Bacteroides fragilis ATCC 25285 |
0.5–2 |
NA |
|
Bacteroides thetaiotaomicron ATCC 29741 |
2–8 |
NA |
|
Eubacterium lentum ATCC 43055 |
0.06–0.25 |
NA |
CLINDAMYCIN HYDROCHLORIDE INDICATIONS AND USAGE
WARNING
Anaerobes:
Streptococci:
Staphylococci:
Pneumococci:
CLINDAMYCIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
WARNING
Clostridium difficileC. difficile
C. difficile C. difficile
C. difficile C. difficile
Usage in Meningitis
PRECAUTIONS
General
Information for Patients
Laboratory Tests
Drug Interactions
in vitro
Carcinogenesis, Mutagenesis, Impairment of Fertility
2
Pregnancy
Teratogenic Effects
Pregnancy Category B
22
Nursing Mothers
Pediatric Use
Geriatric Use
Clostridium difficile
CLINDAMYCIN HYDROCHLORIDE ADVERSE REACTIONS
Gastrointestinal: WARNING WARNINGS
Hypersensitivity Reactions:
Skin and Mucous Membranes: Hypersensitivity Reactions
Liver:
Renal:
Hematopoietic:
Musculoskeletal:
OVERDOSAGE
CLINDAMYCIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
WARNING
Adults
Serious infections More severe infections
Pediatric Patients
Serious infections More severe infections
HOW SUPPLIED
Clindamycin Hydrochloride Capsules USP, 150 mg
Clindamycin Hydrochloride Capsules USP, 300 mg
186
Store at
Pharmacist:
ANIMAL TOXICOLOGY
222
REFERENCES
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement. CLSI document M 100-S20. Wayne, PA: Clinical and Laboratory Standards Institute; 2010.
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Eighth Edition. CLSI document M07-A8. Wayne, PA: Clinical and Laboratory Standards Institute; 2009.
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard - Tenth Edition. CLSI document M02-A10. Wayne, PA: Clinical and Laboratory Standards Institute; 2009.
- CLSI. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard-Seventh Edition. CLSI document M11-A7. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg (100 Capsule Bottle)
NDC 65862-185-01
Clindamycin Hydrochloride Capsules, USP
150 mg*
Rx only 100 Capsules
AUROBINDO

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 300 mg (100 Capsule Bottle)
NDC 65862-186-01
Clindamycin Hydrochloride Capsules, USP
300 mg*
Rx only 100 Capsules
AUROBINDO

Clindamycin HydrochlorideClindamycin Hydrochloride CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clindamycin HydrochlorideClindamycin Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!