Clobetasol Propionate
Renaissance Pharma, Inc.
Denco Asset, LLC
FULL PRESCRIBING INFORMATION: CONTENTS*
- CLOBETASOL PROPIONATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- CLOBETASOL PROPIONATE INDICATIONS AND USAGE
- CLOBETASOL PROPIONATE CONTRAINDICATIONS
- PRECAUTIONS
- General
- Information for Patients
- Laboratory Tests
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Pregnancy
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- CLOBETASOL PROPIONATE ADVERSE REACTIONS
- OVERDOSAGE
- CLOBETASOL PROPIONATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- WARNING
- Principal Display Panel
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Rx only
For Dermatologic Use Only
Not for Ophthalmic Use
CLOBETASOL PROPIONATE DESCRIPTION
Clobetasol propionate foam, 0.05% contains clobetasol propionate, USP, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity.
Clobetasol propionate is pregna-1,4-diene-3,20-dione, 21-chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)-, (11β, 16β)-, with the empirical formula C25H32ClFO5, a molecular weight of 466.97. The following is the chemical structure:
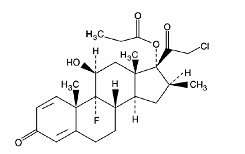
Clobetasol propionate is a white or almost white, odorless, crystalline powder and is insoluble in water.
Clobetasol propionate foam, 0.05% contains 0.5 mg clobetasol propionate, USP, per gram in a thermolabile hydroethanolic foam vehicle consisting of cetyl alcohol, citric acid, ethanol (60%), polysorbate 60, potassium citrate, propylene glycol, purified water, and stearyl alcohol pressurized with a hydrocarbon (propane/butane) propellant.
CLINICAL PHARMACOLOGY
Like other topical corticosteroids, clobetasol propionate foam has anti-inflammatory, antipruritic, and vasoconstrictive properties. The precise mechanism of the anti-inflammatory activity of topical steroids in the treatment of steroid-responsive dermatoses, in general, is uncertain. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Pharmacokinetics
Topical corticosteroids can be absorbed from intact healthy skin. The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusion, inflammation and/or other disease processes in the skin may also increase percutaneous absorption.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Due to the fact that circulating levels are well below the level of detection, the use of pharmacodynamic endpoints for assessing the systemic exposure of topical corticosteroids is necessary. They are metabolized, primarily in the liver, and are then excreted by the kidneys. In addition, some corticosteroids and their metabolites are also excreted in the bile.
CLINICAL STUDIES
A well-controlled clinical study evaluated 188 subjects with moderate to severe scalp psoriasis. Subjects were treated twice daily for 2 weeks with one of four treatments: Clobetasol propionate foam, 0.05%, Vehicle foam, a commercially available clobetasol propionate solution, or Vehicle solution. The efficacy of clobetasol propionate foam, 0.05% in treating scalp psoriasis at the end of the 2 weeks' treatment was superior to that of Vehicle (foam and solution), and was comparable to that of the commercially available Scalp Application. See Table 1 below .
| Clobetasol Propionate Foam, 0.05% n (%) |
Vehicle Foam n (%) |
|
| Total number of subjects |
62 |
31 |
| Subjects with Treatment Success |
39 (63) |
1 (3) |
| Subjects with Parameter Clear at Endpoint (Scalp Psoriasis) |
||
| Scaling - Clear at Endpoint |
42 (68) |
3 (10) |
| Erythema - Clear at Endpoint |
27 (44) |
2 (6) |
| Plaque Thickness - Clear at Endpoint |
41 (66) |
3 (10) |
Another well-controlled clinical study evaluated 279 subjects with mild to moderate plaque-type psoriasis (mean Body Surface Area at baseline was 6.7% with a range from 1% to 20%) of non-scalp regions. Subjects were treated twice daily for 2 weeks with clobetasol propionate foam, 0.05% or Vehicle foam. The face and intertriginous areas were excluded from treatment. The efficacy of clobetasol propionate foam, 0.05% in treating non-scalp psoriasis at the end of 2 weeks' treatment was superior to that of Vehicle foam. See Table 2 below.
| Clobetasol Propionate n (%) |
Vehicle Foam |
|
| Total number of subjects |
139 |
140 |
| Subjects with Treatment Success |
39 (28) |
4 (3) |
| Physician's Static Global Assessment -Clear or Almost Clear at Endpoint |
94 (68) |
30 (21) |
| Scaling -Clear or Almost Clear at Endpoint |
101 (73) |
42 (30) |
| Erythema -Clear or Almost Clear at Endpoint |
88 (63) |
35 (25) |
| Plaque Thickness -Clear at Endpoint |
44 (32) |
5 (4) |
CLOBETASOL PROPIONATE INDICATIONS AND USAGE
Clobetasol propionate foam, 0.05% is a super-potent topical corticosteroid indicated for short-term topical treatment of the inflammatory and pruritic manifestations of moderate to severe corticosteroid- responsive dermatoses of the scalp, and for short-term topical treatment of mild to moderate plaque-type psoriasis on non-scalp regions excluding the face and intertriginous areas.
Treatment beyond 2 consecutive weeks is not recommended and the total dosage should not exceed 50 g per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. In a controlled pharmacokinetic study, some subjects experienced reversible suppression of the adrenals following 14 days of clobetasol propionate foam, 0.05% therapy (See ADVERSE REACTIONS ).
Use in children under 12 years of age is not recommended.
CLOBETASOL PROPIONATE CONTRAINDICATIONS
Clobetasol propionate foam, 0.05% is contraindicated in patients who are hypersensitive to clobetasol propionate, to other corticosteroids, or to any ingredient in this preparation.
PRECAUTIONS
General
Clobetasol propionate is a super-potent topical corticosteroid that has been shown to suppress the adrenals at 7.0 g of clobetasol propionate foam, 0.05% per day. Lesser amounts of clobetasol propionate foam, 0.05% were not studied. Systemic absorption of topical corticosteroids has caused reversible adrenal suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment.
Conditions which augment systemic absorption include the application of more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings.
Patients applying a topical steroid to a large surface area or to areas under occlusion should be evaluated periodically for evidence of adrenal suppression. If adrenal suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid.
Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products.
Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios. See PRECAUTIONS - Pediatric Use .
If irritation develops, clobetasol propionate foam, 0.05% should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than by noting a clinical exacerbation, as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, use of clobetasol propionate foam, 0.05% should be discontinued until the infection has been adequately controlled.
Information for Patients
Patients using topical corticosteroids should receive the following information and instructions:
- 1.This medication is to be used as directed by the physician and should not be used longer than the prescribed time period. It is for external use only. Avoid contact with the eyes.
- 2.This medication should not be used for any disorder other than that for which it was prescribed.
- 3.The treated area should not be bandaged or otherwise covered or wrapped so as to be occlusive unless directed by the physician.
- 4.Patients should report to their physician any signs of local adverse reactions.
Laboratory Tests
The following tests may be helpful in evaluating patients for adrenal suppression:
ACTH stimulation test
A.M. plasma cortisol test
Urinary free cortisol test
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of clobetasol propionate.
Clobetasol propionate was non-mutagenic in three different test systems: the Ames test, the Saccharomycescerevisiae gene conversion assay, and the E. coli B WP2 fluctuation test.
Studies in the rat following subcutaneous administration of clobetasol propionate at dosage levels up to 0.05 mg/kg per day revealed that the females exhibited an increase in the number of resorbed embryos and a decrease in the number of living fetuses at the highest dose.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application to laboratory animals.
Clobetasol propionate has not been tested for teratogenicity by the topical route; however, it is absorbed percutaneously, and when administered subcutaneously, it was a significant teratogen in both the rabbit and the mouse. Clobetasol propionate has greater teratogenic potential than steroids that are less potent.
Teratogenicity studies in mice using the subcutaneous route resulted in fetotoxicity at the highest dose tested (1 mg/kg) and teratogenicity at all dose levels tested down to 0.03 mg/kg. These doses are approximately 1.4 and 0.04 times, respectively, the human topical dose of clobetasol propionate foam, 0.05% based on body surface area comparisons. Abnormalities seen included cleft palate and skeletal abnormalities.
In rabbits, clobetasol propionate was teratogenic at doses of 0.003 and 0.01 mg/kg. These doses are approximately 0.02 and 0.05 times, respectively, the human topical dose of clobetasol propionate foam, 0.05% based on body surface area comparisons. Abnormalities seen included cleft palate, cranioschisis, and other skeletal abnormalities.
There are no adequate and well-controlled studies of the teratogenic potential of clobetasol propionate in pregnant women. Clobetasol propionate foam, 0.05% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Because many drugs are excreted in human milk, caution should be exercised when clobetasol propionate foam, 0.05% is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of clobetasol propionate foam, 0.05% in pediatric patients have not been established; therefore, use in children under 12 years of age is not recommended. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of adrenal suppression and Cushing's syndrome when they are treated with topical corticosteroids. Pediatric patients are therefore at greater risk of adrenal insufficiency during and/or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children.
Adrenal suppression, Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Geriatric Use
Clinical studies of clobetasol propionate foam, 0.05% did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
CLOBETASOL PROPIONATE ADVERSE REACTIONS
In a controlled pharmacokinetic study, 5 of 13 subjects experienced reversible suppression of the adrenals at any time during the 14 days of clobetasol propionate foam, 0.05% therapy to at least 20% of the body surface area. Of the 13 subjects studied, 1 of 9 with psoriasis were suppressed after 14 days and all 4 of the subjects with atopic dermatitis had abnormal cortisol levels indicative of adrenal suppression at some time after starting therapy with clobetasol propionate foam, 0.05%. See Table 3 below.
| Dermatosis |
Clobetasol Propionate Foam, 0.05% |
| Psoriasis |
1 of 9 |
| Atopic Dermatitis |
4 of 4 |
Systemic absorption of topical corticosteroids has produced reversible adrenal suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in some patients. See PRECAUTIONS.
In a controlled clinical trial (188 subjects) with clobetasol propionate foam, 0.05% in subjects with psoriasis of the scalp, there were no localized scalp adverse reactions reported in the clobetasol propionate foam, 0.05% treated subjects. In two controlled clinical trials (360 subjects) with clobetasol propionate foam, 0.05% in subjects with psoriasis of non-scalp regions, localized adverse events that occurred in the clobetasol propionate foam, 0.05% treated subjects included application site burning (10%), application site dryness (<1%), and other application site reactions (4%).
In larger controlled trials with other clobetasol propionate formulations, the most frequently reported local adverse reactions have included burning, stinging, irritation, pruritus, erythema, folliculitis, cracking and fissuring of the skin, numbness of the fingers, skin atrophy, and telangiectasia (all less than 2%).
The following additional local adverse reactions have been reported with topical corticosteroids, but they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids such as clobetasol propionate foam, 0.05%. These reactions are listed in an approximate decreasing order of occurrence: dryness, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, striae, and miliaria.
OVERDOSAGE
Topically applied clobetasol propionate foam, 0.05% can be absorbed in sufficient amounts to produce systemic effects. See PRECAUTIONS.
CLOBETASOL PROPIONATE DOSAGE AND ADMINISTRATION
Note: For proper dispensing of foam, hold the can upside down and depress the actuator.
Clobetasol propionate foam, 0.05% should be applied to the affected area twice daily, once in the morning and once at night. Invert the can and dispense a small amount of clobetasol propionate foam, 0.05% (up to a maximum of a golf-ball-size dollop or one and a half capfuls) into the cap of the can, onto a saucer or other cool surface, or to the lesion, taking care to avoid contact with the eyes. Dispensing directly onto hands is not recommended (unless the hands are the affected area), as the foam will begin to melt immediately upon contact with warm skin. When applying clobetasol propionate foam, 0.05% to a hair-bearing area, move the hair away from the affected area so that the foam can be applied to each affected area. Pick up small amounts with fingertips and gently massage into affected area until the foam disappears. Repeat until entire affected area is treated.
Apply the smallest amount possible that sufficiently covers the affected area(s). No more than one and a half capfuls of foam should be used at each application. Do not apply to face or intertriginous areas.
Clobetasol propionate foam, 0.05% is a super-high-potency topical corticosteroid; therefore, treatment should be limited to 2 consecutive weeks and amounts greater than 50 g/week should not be used. Use in pediatric patients under 12 years of age is not recommended.
Unless directed by a physician, clobetasol propionate foam, 0.05% should not be used with occlusive dressings.
| Apply clobetasol propionate foam, 0.05% twice a day, once in the morning and once at night. Apply only enough to cover the affected areas. Clobetasol propionate foam, 0.05% should not be applied to the groin, or other skin fold areas. |
|

|
Before applying clobestasol propionate foam, 0.05% for the first time, break the tiny plastic piece at the base of the can's rim by gently pushing back (away from the piece) on the nozzle. |

|
Turn the can upside down. Push the button to squirt a small amount of clobetasol propionate foam, 0.05% into the cap of the can, onto a saucer or other cool surface, or your affected skin area. This amount should be no more than 1 1/2 capfuls, about the size of a golf ball. Do not squirt clobetasol propionate foam, 0.05% directly onto your hands (unless your hands are the affected areas), because the foam will begin to melt right away on contact with your warm skin. If your fingers are warm, rinse them in cold water first. (Be sure to dry them thoroughly before handling the foam.) If the can seems warm or the foam seems runny, run the can under cold water. |

|
Using your fingertips, gently massage clobetasol propionate foam, 0.05% into the affected areas until the foam disappears. If you are treating areas with hair such as the scalp, move any hair away so that the foam can be applied directly to the affected areas. Repeat the process until the affected areas are treated. Keep the foam away from your eyes, as it will sting and may cause eye problems if there is frequent contact with your eyes. If the foam gets your eyes, rinse them well with cold water right away. If the stinging continues, contact your doctor right away. |

|
Wash your hands after apply clobetasol propionate foam, 0.05%. Throw away any of the unused medicine that you squirted out of the can. |
HOW SUPPLIED
Clobetasol Propionate Foam is supplied as follows:
- 50 g aluminum can NDC 40085-888-50
- 100 g aluminum can NDC 40085-888-00
Store at controlled room temperature 68-77ºF (20-25ºC).
WARNING
FLAMMABLE. AVOID FIRE, FLAME OR SMOKING DURING AND IMMEDIATELY FOLLOWING APPLICATION. Keep out of reach of children. Contents under pressure. Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120ºF (49ºC).
Manufactured for
Renaissance Pharma, Inc.
Newtown, PA 18940
by DPT Laboratories, Ltd
San Antonio, TX 78215
For additional information:
1-866-897-5002
October 2013 140102-0613
Principal Display Panel
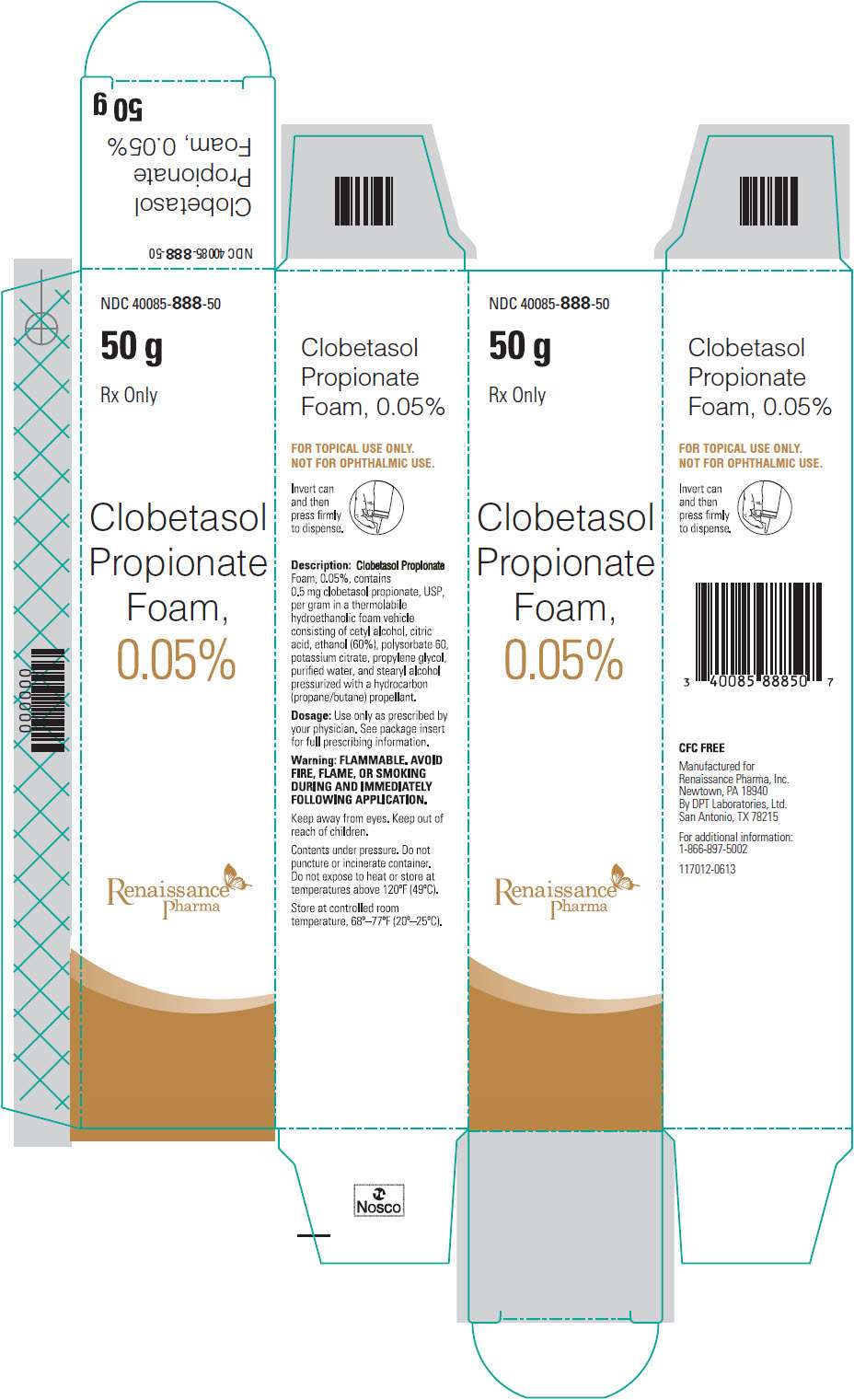
NDC 40085-888-50
50 g
Rx Only
Clobetasol Propionate Foam, 0.05%
Renaissance Pharma
FOR TOPICAL USE ONLY.
NOT FOR OPHTHALMIC USE.
Invert can and then press firmly to dispense.
Description: Clobetasol Propionate Foam, 0.05% contains 0.5 mg clobetasol propionate, USP, per gram in a thermolabile hydroethanolic foam vehicle consisting of cetyl alcohol, citric acid, ethanol (60%), polysorbate 60, potassium citrate, propylene glycol, purified water, and stearyl alcohol pressurized with a hydrocarbon (propane/butane) propellant.
Dosage: Use only as prescribed by your physician. See package insert for full prescribing information.
Warning: FLAMMABLE. AVOID FIRE, FLAME, OR SMOKING DURING AND IMMEDIATELY FOLLOWING APPLICATION.
Keep away from eyes. Keep out of reach of children.
Contents under pressure. Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120oF (49oC).
Store at controlled room temperature, 68o – 77oF (20o – 25oC).
CFC FREE
Manufactured for
Renaissance Pharma, Inc.
Newtown, PA 18940
By DPT Laboratories, Ltd.
San Antonio, TX 78215
For additional information:
1-866-897-5002
117012-0613
Principal Display Panel
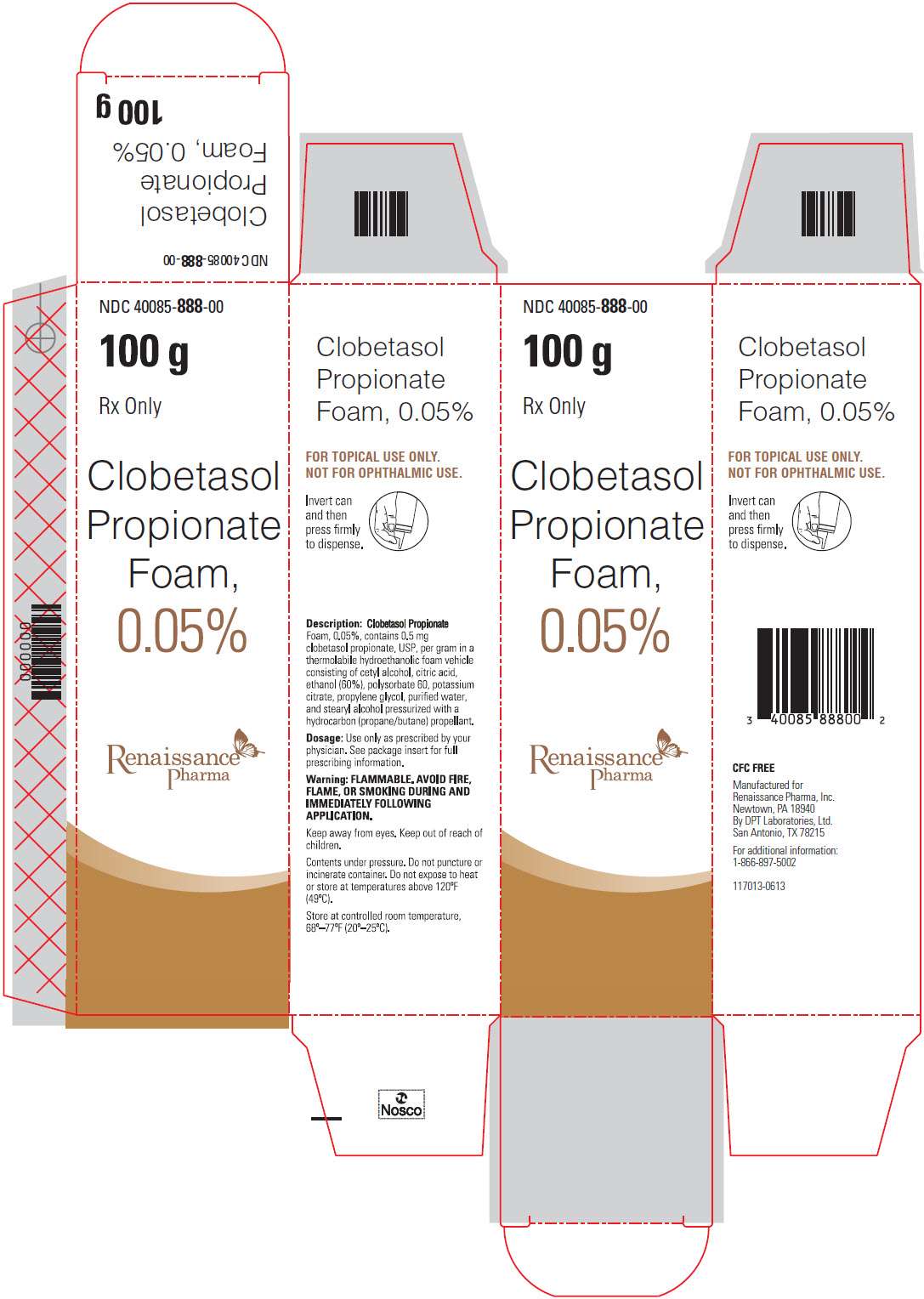
NDC 40085-888-00
100 g
Rx Only
Clobetasol Propionate Foam, 0.05%
Renaissance Pharma
FOR TOPICAL USE ONLY.
NOT FOR OPHTHALMIC USE.
Invert can and then press firmly to dispense.
Description: Clobetasol Propionate Foam, 0.05% contains 0.5 mg clobetasol propionate, USP, per gram in a thermolabile hydroethanolic foam vehicle consisting of cetyl alcohol, citric acid, ethanol (60%), polysorbate 60, potassium citrate, propylene glycol, purified water, and stearyl alcohol pressurized with a hydrocarbon (propane/butane) propellant.
Dosage: Use only as prescribed by your physician. See package insert for full prescribing information.
Warning: FLAMMABLE. AVOID FIRE, FLAME, OR SMOKING DURING AND IMMEDIATELY FOLLOWING APPLICATION.
Keep away from eyes. Keep out of reach of children.
Contents under pressure. Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120oF (49oC).
Store at controlled room temperature, 68o – 77oF (20o – 25oC).
CFC FREE
Manufactured for
Renaissance Pharma, Inc.
Newtown, PA 18940
By DPT Laboratories, Ltd.
San Antonio, TX 78215
For additional information:
1-866-897-5002
117013-0613
Clobetasol Propionateclobetasol propionate AEROSOL, FOAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
