CONDITION AND ENHANCE SYSTEM
Obagi Condition & Enhance Clear (Hydroquinone USP, 4%) Skin Bleaching Cream Obagi Condition & Enhance Blender (Hydroquinone USP, 4%) Skin Bleaching Cream
FULL PRESCRIBING INFORMATION: CONTENTS*
- CONDITION AND ENHANCE SYSTEM DESCRIPTION
- CLINICAL PHARMACOLOGY
- CONDITION AND ENHANCE SYSTEM INDICATIONS AND USAGE
- CONDITION AND ENHANCE SYSTEM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CONDITION AND ENHANCE SYSTEM ADVERSE REACTIONS
- CONDITION AND ENHANCE SYSTEM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - Kit Carton
FULL PRESCRIBING INFORMATION
Rx Only
FOR EXTERNAL USE ONLY
CONDITION AND ENHANCE SYSTEM DESCRIPTION
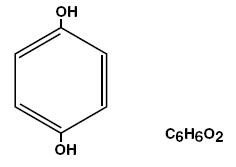
Hydroquinone is 1,4-benzenediol. Hydroquinone occurs as fine, white needles. The drug is freely soluble in water and in alcohol. Chemically, hydroquinone is designated as p-dihydroxybenzene; the empirical formula is C6 H6 O2; molecular weight is 110.0.
Obagi® Condition & Enhance Blender contains Hydroquinone USP 40 mg/gm in a base of purified water, glycerin, cetyl alcohol, PPG-2 myristyl ether propionate, sodium lauryl sulfate, TEA-salicylate, lactic acid, phenyl trimethicone, tocopheryl acetate, sodium metabisulfite, ascorbic acid, methylparaben, saponins, disodium EDTA, BHT, and propylparaben.
Obagi® Condition & Enhance Clear contains Hydroquinone USP 40 mg/gm in a base of purified water, cetyl alcohol, glycerin, sodium lauryl sulfate, stearyl alcohol, tocopheryl acetate, ascorbic acid, sodium metabisulfite, lactic acid, saponins, disodium EDTA, methylparaben, BHT, propylparaben, and butylparaben.
CLINICAL PHARMACOLOGY
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3, 4-dihydroxyphenylalanine (dopa) and suppression of other melanocyte metabolic processes.
Exposure to sunlight or ultraviolet light will cause repigmentation of the bleached areas, which may be prevented by the use of sunblocking agents or sunscreen agents contained in Obagi Condition & Enhance.
CONDITION AND ENHANCE SYSTEM INDICATIONS AND USAGE
The gradual bleaching of hyperpigmented skin conditions such as chloasma, melasma, freckles, senile lentigines, and other unwanted areas of melanin hyperpigmentation.
CONDITION AND ENHANCE SYSTEM CONTRAINDICATIONS
Prior history of sensitivity or allergic reaction to this product or any of its ingredients. The safety of topical hydroquinone use during pregnancy or in children (12 years and under) has not been established.
WARNINGS
Caution
Hydroquinone is a skin bleaching agent which may produce unwanted cosmetic effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this medication.
Test for skin sensitivity before using by applying a small amount to an unbroken patch of skin and check in 24 hours. Minor redness is not a contraindication, but where there is itching or vesicle formation or excessive inflammatory response, further treatment is not advised. Close patient supervision is recommended.
Avoid contact with eyes. In case of accidental contact, patient should rinse eyes thoroughly with water and contact physician. A bitter taste and anesthetic effect may occur if applied to lips.
Sunscreen use is an essential aspect of hydroquinone therapy because even minimal sunlight exposure sustains melanocytic activity.
Warning
Contains sodium metabisulfite, a sulfite that may cause serious allergic type reactions (e.g., hives, itching, wheezing, anaphylaxis, severe asthma attacks) in certain susceptible persons.
PRECAUTIONS
(SEE WARNINGS)
General
Treatment should be limited to relatively small areas of the body at one time since some patients experience a transient skin reddening and a mild burning sensation which does not preclude treatment.
Pregnancy Category C
Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether hydroquinone can cause fetal harm when used topically on a pregnant woman or affect reproductive capacity. It is not known to what degree, if any, topical hydroquinone is absorbed systemically. Topical hydroquinone should be used on pregnant women only when clearly indicated.
Nursing mothers
It is not known whether topical hydroquinone is absorbed or excreted in human milk. Caution is advised when topical hydroquinone is used by a nursing mother.
Pediatric usage
Safety and effectiveness in children below the age of 12 years have not been established.
CONDITION AND ENHANCE SYSTEM ADVERSE REACTIONS
No systemic adverse reactions have been reported. Occasional hypersensitivity (localized contact dermatitis) may occur, in which case the medication should be discontinued and the physician notified immediately.
CONDITION AND ENHANCE SYSTEM DOSAGE AND ADMINISTRATION
A thin application should be applied to the affected area twice daily or as directed by a physician. If no improvement is seen after three (3) months of treatment, use of this product should be discontinued. Sun exposure should be limited by using a sunscreen agent, a sun blocking agent, or protective clothing to cover bleached skin when using and after using this product in order to prevent repigmentation.
HOW SUPPLIED
Obagi Condition and Enhance Blender is available as follows:
| 2 oz. (57 gm) bottle | NDC 62032-115-36 |
| 1 oz. (28.5 gm) bottle | NDC 62032-115-10 |
Obagi Condition and Enhance Clear is available as follows:
| 2 oz. (57 gm) bottle | NDC 62032-117-36 |
Store at 25°C (77°F); excursion permitted to 15°C-30°C (59°F-86°F).
OBAGI
®
MEDICAL
OMP, Inc.
Long Beach, CA 90802
USA
1-800-636-7546
80707910U Rev. 6/07
PRINCIPAL DISPLAY PANEL - Kit Carton
OBAGI
®
CONDITION & ENHANCE SYSTEM
For Use with Surgical Procedures
Travel Size

CONDITION AND ENHANCE SYSTEMHYDROQUINONE, OCTINOXATE AND ZINC OXIDE KIT
| ||||||||||||||||||||||||||||||||||||||||