Curosurf
CUROSURF (poractant alfa)Intratracheal Suspension
FULL PRESCRIBING INFORMATION: CONTENTS*
- CUROSURF DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATION AND USAGE
- WARNINGS
- PRECAUTIONS
- CUROSURF ADVERSE REACTIONS
- FOLLOW-UP EVALUATIONS
- OVERDOSAGE
- CUROSURF DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
CUROSURF DESCRIPTION
CUROSURF® (poractant alfa) Intratracheal Suspension is a sterile, non-pyrogenic pulmonary surfactant intended for intratracheal use only. It is an extract of natural porcine lung surfactant consisting of 99% polar lipids (mainly phospholipids) and 1% hydrophobic low molecular weight proteins (surfactant associated proteins SP-B and SP-C).
It is suspended in 0.9% sodium chloride solution. The pH is adjusted as required with sodium bicarbonate to a pH of 6.2 (5.5 - 6.5).
CUROSURF contains no preservatives.
CUROSURF is a white to creamy white suspension of poractant alfa. Each milliliter of suspension contains 80 mg of surfactant (extract) that includes 76 mg of phospholipids and 1 mg of protein of which 0.45 mg is SP-B. The amount of phospholipids is calculated from the content of phosphorus and contains 55 mg of phosphatidylcholine of which 30 mg is dipalmitoylphosphatidylcholine.
CLINICAL PHARMACOLOGY
Mechanism of Action
Endogenous pulmonary surfactant reduces surface tension at the air-liquid interface of the alveoli during ventilation and stabilizes the alveoli against collapse at resting transpulmonary pressures.
A deficiency of pulmonary surfactant in preterm infants results in Respiratory Distress Syndrome (RDS) characterized by poor lung expansion, inadequate gas exchange, and a gradual collapse of the lungs (atelectasis).
CUROSURF compensates for the deficiency of surfactant and restores surface activity to the lungs of these infants.
Activity
In vitro - CUROSURF lowers minimum surface tension to ≤4mN/m as measured by the Wilhelmy Balance System.
In vivo - In several pharmacodynamic studies, CUROSURF improved lung compliance, pulmonary gas exchange, or survival in premature rabbits.
Pharmacokinetics
CUROSURF is administered directly to the target organ, the lung, where biophysical effects occur at the alveolar surface.
No human pharmacokinetic studies to characterize the absorption, biotransformation, or excretion of CUROSURF have been performed. Non-clinical studies have been performed to evaluate the disposition of phospholipids present in CUROSURF.
Animal Metabolism
In both adult and newborn rabbits, approximately 50% of the radiolabeled component was rapidly removed from the alveoli in the first three hours after single intratracheal administration of CUROSURF-14C-DPPC (dipalmitoylphosphatidylcholine).
Over a 24-hour period, approximately 45% of the labeled DPPC was cleared from the lungs of adult rabbits compared to approximately 20% in newborn rabbits.
In newborn rabbits, CUROSURF-14C-DPPC passed from the alveolar space into the lung parenchyma and then was secreted again into the alveoli, whereas in adult rabbits, most of the DPPC was not recycled. The half-life in the lung appeared to be about 25 hours in adult rabbits and 67 hours in newborn rabbits.
The concentration of 14C-DPPC in alveolar macrophages was ≤2% of that in the lung in newborn and adult rabbits. Of the total 14C-DPPC recovered in newborn rabbits, <0.6% was found in the serum, liver, kidneys, and brain, respectively, at 48 hours.
No information is available about the metabolic rate of the surfactant-associated proteins in CUROSURF.
CLINICAL STUDIES
The clinical efficacy of CUROSURF was demonstrated in one single-dose study (Study 1) and one multiple-dose study (Study 2) in the treatment of established neonatal RDS involving approximately 500 infants. Each study was randomized, multicenter, and controlled. In Study 1, infants 700-2000 g birth weight with RDS requiring mechanical ventilation and a FiO2≥0.60 were enrolled.
CUROSURF 2.5 mL/kg single dose (200 mg/kg) or control (disconnection from the ventilator and manual ventilation for 2 minutes) was administered after RDS developed and before 15 hours of age.
The results from Study 1 are shown below in Table 1.
| EFFICACY PARAMETER | SINGLE-DOSE CUROSURF n=78 % |
CONTROL n=67 % |
P-VALUE |
|---|---|---|---|
| N.S.: not statistically significant | |||
| MORTALITY at 28 DAYS (ALL CAUSES) |
31 | 48 | ≤0.05 |
BRONCHOPULMONARY DYSPLASIA |
18 | 22 | N.S. |
| PNEUMOTHORAX | 21 | 36 | ≤0.05 |
| PULMONARY INTERSTITIAL EMPHYSEMA | 21 | 38 | ≤0.05 |
In Study 2, infants 700-2000g birth weight with RDS requiring mechanical ventilation and a FiO2≥0.60 were enrolled.
In this two-arm trial, CUROSURF was administered after RDS developed and before 15 hours of age, as a single-dose or as multiple doses.
In the single-dose arm, infants received CUROSURF 2.5mL/kg (200mg/kg). In the multiple-dose arm, the initial dose of CUROSURF was 2.5mL/kg (200mg/kg) and subsequent doses of CUROSURF were 1.25mL/kg (100mg/kg). The results from Study 2 are shown below in Table 2.
| EFFICACY PARAMETER | SINGLE-DOSE CUROSURF n=184 % |
MULTIPLE-DOSE CUROSURF n=173 % |
P-VALUE |
|---|---|---|---|
| N.S.: not statistically significant | |||
| MORTALITY at 28 DAYS (ALL CAUSES) | 21 | 13 | 0.048 |
| BRONCHOPULMONARY DYSPLASIA | 18 | 18 | N.S. |
| PNEUMOTHORAX | 17 | 9 | 0.03 |
| PULMONARY INTERSTITIAL EMPHYSEMA | 27 | 22 | N.S. |
ACUTE CLINICAL EFFECTS
As with other surfactants, marked improvements in oxygenation may occur within minutes of the administration of CUROSURF.
INDICATION AND USAGE
CUROSURF is indicated for the treatment (rescue) of Respiratory Distress Syndrome (RDS) in premature infants. CUROSURF reduces mortality and pneumothoraces associated with RDS.
WARNINGS
CUROSURF is intended for intratracheal use only.
THE ADMINISTRATION OF EXOGENOUS SURFACTANTS, INCLUDING CUROSURF, CAN RAPIDLY AFFECT OXYGENATION AND LUNG COMPLIANCE. Therefore, infants receiving CUROSURF should receive frequent clinical and laboratory assessments so that oxygen and ventilatory support can be modified to respond to respiratory changes. CUROSURF should only be administered by those trained and experienced in the care, resuscitation, and stabilization of pre-term infants.
TRANSIENT ADVERSE EFFECTS SEEN WITH THE ADMINISTRATION OF CUROSURF INCLUDE BRADYCARDIA, HYPOTENSION, ENDOTRACHEAL TUBE BLOCKAGE, AND OXYGEN DESATURATION. These events require stopping Curosurf administration and taking appropriate measures to alleviate the condition. After the patient is stable, dosing may proceed with appropriate monitoring.
PRECAUTIONS
General
Correction of acidosis, hypotension, anemia, hypoglycemia, and hypothermia is recommended prior to CUROSURF administration. Surfactant administration can be expected to reduce the severity of RDS but will not eliminate the mortality and morbidity associated with other complications of prematurity.
Sufficient information is not available on the effects of administering initial doses of CUROSURF other than 2.5 mL/kg (200 mg/kg), subsequent doses other than 1.25 mL/kg (100 mg/kg), administration of more than three total doses, dosing more frequently than every 12 hours, or initiating therapy with CUROSURF more than 15 hours after diagnosing RDS. Adequate data are not available on the use of CUROSURF in conjunction with experimental therapies of RDS, e.g., high-frequency ventilation.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to assess potential carcinogenic and reproductive effects of CUROSURF, or other surfactants, have not been conducted.
Mutagenicity studies of CUROSURF, which included the Ames test, gene mutation assay in Chinese hamster V79 cells, chromosomal aberration assay in Chinese hamster ovarian cells, unscheduled DNA synthesis in HELA S3 cells, and in vivo mouse nuclear test, were negative.
CUROSURF ADVERSE REACTIONS
Transient adverse effects seen with the administration of CUROSURF include bradycardia, hypotension, endotracheal tube blockage, and oxygen desaturation. Pulmonary Hemmorhage is a known complication of premature birth and very low birth-weight and has been reported both in clinical trials with Curosurf and in post-marketing adverse event reports in infants who had received Curosurf. The rates of common complications of prematurity observed in Study 1 are shown below in Table 3.
| COMPLICATIONS OF PREMATURITY | ||
|---|---|---|
| CUROSURF 2.5 mL/kg (200 mg/kg) n=78 % |
CONTROL * n=66 % |
|
| Acquired Pneumonia | 17 | 21 |
| Acquired Septicemia | 14 | 18 |
| Bronchopulmonary Dysplasia | 18 | 22 |
| Intracranial Hemorrhage | 51 | 64 |
| Patent Ductus Arteriosus | 60 | 48 |
| Pneumothorax | 21 | 36 |
| Pulmonary Interstitial Emphysema | 21 | 38 |
Immunological studies have not demonstrated differences in levels of surfactant-anti-surfactant immune complexes and anti-CUROSURF antibodies between patients treated with CUROSURF and patients who received control treatment.
FOLLOW-UP EVALUATIONS
Seventy-six infants (45 treated with CUROSURF) were evaluated at 1 year of age and 73 infants (44 treated with CUROSURF) at 2 years of age. Data from follow-up evaluations for weight and length, persistent respiratory symptoms, incidence of cerebral palsy, visual impairment, or auditory impairment was similar between treatment groups. In 16 patients (10 treated with CUROSURF and 6 controls) evaluated at 5.5 years of age, the developmental quotient, derived using the Griffiths Mental Developmental Scales, was similar between groups.
OVERDOSAGE
There have been no reports of overdosage following the administration of CUROSURF. In the event of accidental overdosage, and only if there are clear clinical effects on the infant's respiration, ventilation, or oxygenation, as much of the suspension as possible should be aspirated and the infant should be managed with supportive treatment, with particular attention to fluid and electrolyte balance.
CUROSURF DOSAGE AND ADMINISTRATION
FOR INTRATRACHEAL ADMINISTRATION ONLY.
General
CUROSURF is administered intratracheally by instillation through a 5 French end-hole catheter, and briefly disconnecting the endotracheal tube from the ventilator. Alternatively, CUROSURF may be administered through the secondary lumen of a dual lumen endotracheal tube without interrupting mechanical ventilation.
Before administering CUROSURF, assure proper placement and patency of the endotracheal tube. At the discretion of the clinician, the endotracheal tube may be suctioned before administering Curosurf. The infant should be allowed to stabilize before proceeding with dosing.
The initial recommended dose of CUROSURF is 2.5 mL/kg birth weight. This dose may be determined from the CUROSURF dosing chart below.
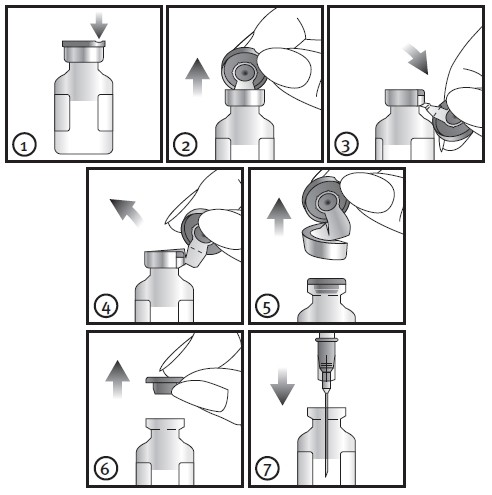
Slowly withdraw the entire contents of the vial of CUROSURF into a 3 or 5 mL plastic syringe through a large-gauge needle (e.g., at least 20 gauge). Attach the pre-cut 8-cm 5 end-hole French catheter to the syringe. Fill the catheter with CUROSURF. Discard excess CUROSURF through the catheter so that only the total dose to be given remains in the syringe.
Immediately before CUROSURF administration, the infant's ventilator settings should be changed to a rate of 40-60 breaths/minute, inspiratory time 0.5 second, and supplemental oxygen sufficient to maintain SaO2>92%. Keep the infant in a neutral position (head and body in alignment without inclination). Briefly disconnect the endotracheal tube from the ventilator. Insert the pre-cut 5 French catheter into the endotracheal tube and instill the first aliquot (1.25 mL/kg birth weight) of CUROSURF. The infant should be positioned such that either the right or left side is dependent for this aliquot. After the first aliquot is instilled, remove the catheter from the endotracheal tube and manually ventilate the infant with 100% oxygen at a rate of 40-60 breaths/minute for one minute. When the infant is stable, reposition the infant such that the other side is dependant and administer the remaining aliquot using the same procedures. Do not suction airways for 1 hour after surfactant instillation unless signs of significant airway obstruction occur. After completion of the dosing procedure, resume usual ventilator management and clinical care. In the clinical trials, ventilator management was modified to maintain a PaO2 of about 55 mmHg, PaCO2 of 35-45, and pH >7.3.
Slowly withdraw the entire contents of the vial of CUROSURF into a 3 or 5 mL plastic syringe through a large-gauge needle (e.g., at least 20 gauge). Do not attach 5 French end-hole catheter. Keep the infant in a neutral position (head and body in alignment without inclination). Administer CUROSURF through the proximal end of the secondary lumen of the endotracheal tube as a single dose, given over 1 minute, and without interrupting mechanical ventilation. After completion of this dosing procedure, ventilatory management may require transient increases in FiO2, ventilatory rate, or PIP.
Repeat doses
Up to two repeat doses of 1.25 mL/kg birth weight each may be administered, using the same techniques described for the initial dose. Repeat doses should be administered, at approximately 12-hour intervals, in infants who remain intubated and in whom RDS is considered responsible for their persisting or deteriorating respiratory status. The maximum recommended total dose (sum of the initial and up to two repeat doses) is 5 mL/kg.
| CUROSURF DOSING CHART | |||||
|---|---|---|---|---|---|
| WEIGHT (grams) | INITIAL DOSE 2.5mL/kg | REPEAT DOSE 1.25mL/kg | WEIGHT (grams) | INITIAL DOSE 2.5mL/kg | REPEAT DOSE 1.25mL/kg |
| EACH DOSE (mL) | EACH DOSE (mL) | ||||
| 600-650 | 1.60 | 0.80 | 1301-1350 | 3.30 | 1.65 |
| 651-700 | 1.70 | 0.85 | 1351-1400 | 3.50 | 1.75 |
| 701-750 | 1.80 | 0.90 | 1401-1450 | 3.60 | 1.80 |
| 751-800 | 2.00 | 1.00 | 1451-1500 | 3.70 | 1.85 |
| 801-850 | 2.10 | 1.05 | 1501-1550 | 3.80 | 1.90 |
| 851-900 | 2.20 | 1.10 | 1551-1600 | 4.00 | 2.00 |
| 901-950 | 2.30 | 1.15 | 1601-1650 | 4.10 | 2.05 |
| 951-1000 | 2.50 | 1.25 | 1651-1700 | 4.20 | 2.10 |
| 1001-1050 | 2.60 | 1.30 | 1701-1750 | 4.30 | 2.15 |
| 1051-1100 | 2.70 | 1.35 | 1751-1800 | 4.50 | 2.25 |
| 1101-1150 | 2.80 | 1.40 | 1801-1850 | 4.60 | 2.30 |
| 1151-1200 | 3.00 | 1.50 | 1851-1900 | 4.70 | 2.35 |
| 1201-1250 | 3.10 | 1.55 | 1901-1950 | 4.80 | 2.40 |
| 1251-1300 | 3.20 | 1.60 | 1951-2000 | 5.00 | 2.50 |
Directions for Use
CUROSURF should be inspected visually for discoloration prior to administration. The color of CUROSURF is white to creamy white. CUROSURF should be stored in a refrigerator at +2 to +8°C (36-46°F). Before use, the vial should be slowly warmed to room temperature and gently turned upside-down, in order to obtain a uniform suspension. DO NOT SHAKE.
Unopened, unused vials of CUROSURF that have warmed to room temperature can be returned to refrigerated storage within 24 hours for future use.
Do not warm to room temperature and return to refrigerated storage more than once. Protect from light. Each single-use vial should be entered only once and the vial with any unused material should be discarded after the initial entry.
Dosing Precautions
Transient episodes of bradycardia, decreased oxygen saturation, reflux of the surfactant into the endotracheal tube, and airway obstruction have occurred during the dosing procedure of CUROSURF.
These events require interrupting the administration of CUROSURF and taking the appropriate measures to alleviate the condition. After stabilization, dosing may resume with appropriate monitoring.
HOW SUPPLIED
CUROSURF® (poractant alfa) Intratracheal Suspension (NDC Numbers: 10122-510-01 [1.5 mL]; 10122-510-03 [3 mL]) is available in sterile, ready-to-use rubber-stoppered clear glass vials containing 1.5 mL [120 mg surfactant (extract) or 3 mL (240 mg surfactant (extract)] of suspension. One vial per carton.
Store CUROSURF Intratracheal Suspension in a refrigerator at +2 to +8°C (36-46°F). Unopened vials of CUROSURF may be warmed to room temperature for up to 24 hours prior to use.
CUROSURF should not be warmed to room temperature and returned to the refrigerator more than once. PROTECT FROM LIGHT. Do not shake. Vials are for single use only. After opening the vial discard the unused portion of the drug.
Rx only.
Manufactured for:
Cornerstone Therapeutics Inc., Cary, NC 27518
Manufactured by and licensed from:
Chiesi Farmaceutici, S.p.A.
Parma, Italy 43100
CTC1400C0410-SPL
82W03.09/01


Curosurfporactant alfa SUSPENSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||