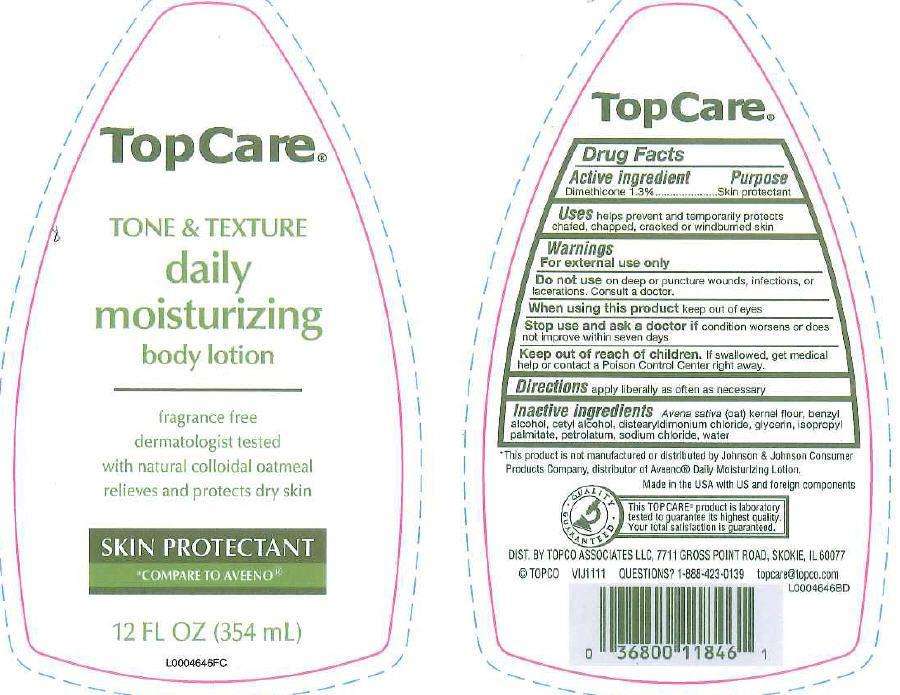
Daily Moisturizing
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Daily Moisturizing Uses
- Warnings
- Do not use
- When using this product
- Stop use
- Keep out of reach of children
- Directions
- Inactive ingredients
- Disclaimer
- Side Effects
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredient
Dimethicone 1.3%
Purpose
Purpose
Skin Protectant
Uses
Uses helps prevent and temprarily protects chafed, chapped, cracked or windburned skin.
Warnings
Warnings
For external use only
Do not use
Do not use on deep or puncture wounds, infections, or lacerations. Consult a doctor
When using this product
Wen using this product keep out of eyes
Stop use
Stop use and ask a doctor if condition worsens or does not improve within seven days
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
Directions apply liberally as often at necessary
Inactive ingredients
Inactive ingredients avena sativa (oat)kernel flour, benzyl alcohol, cetyl alcohol, distearyldimonium chloride, glycerin, isopropyl palmitate, petrolaturm, sodium chloride, water
Disclaimer
This product is not manufactured or distributed by Johnon + Johnson Consumer Products Company, distributor of Aveno Daily Moisturizing Lotion
Made in the USA with US and foreign components
Side Effects
DIST. BY TOPCO ASSOCIATES LLC, 7711 GROSS POINT ROADK,
SKOKIE 60077
QUESTIONS? 1-888-423-0139 topcare@topco.com
Principal Display Panel
TopCare
TONE + TEXTURE
daily
moisturizing
body lotion
fragrance free
dermatologist tested
with natural colloidal oatmeal
relieves and protects dry skin
SKIN PROTECTANT
COMPARE TO AVEENO
12 FL OZ (354 mL)
Daily MoisturizingDimethicone LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||