DAPTACEL
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DAPTACEL safely and effectively. See full prescribing information for DAPTACEL. DAPTACEL (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed) Suspension for Intramuscular InjectionInitial U.S. Approval: 2002RECENT MAJOR CHANGES Warnings & Precautions (5.8) 10/2013 INDICATIONS AND USAGE DAPTACEL is a vaccine indicated for active immunization against diphtheria, tetanus and pertussis as a five dose series in infants and children 6 weeks through 6 years of age (prior to 7th birthday). (1) DOSAGE AND ADMINISTRATION The five dose immunization series consists of a 0.5 mL intramuscular injection administered at 2, 4, 6 and 15-20 months of age, and at 4-6 years of age. (2.1, 2.2) DOSAGE FORMS AND STRENGTHS Suspension for injection, supplied in single dose (0.5 mL) vials (3) CONTRAINDICATIONS Severe allergic reaction (e.g. anaphylaxis) after a previous dose of any diphtheria toxoid, tetanus toxoid, or pertussis-containing vaccine, or any component of DAPTACEL. (4.1) Encephalopathy within 7 days of a previous pertussis-containing vaccine with no other identifiable cause. (4.2) Progressive neurologic disorder until a treatment regimen has been established and the condition has stabilized. (4.3) WARNINGS AND PRECAUTIONS Carefully consider benefits and risks before administering DAPTACEL to persons with a history of: -fever ≥40.5°C (105°F), hypotonic-hyporesponsive episode (HHE) or persistent, inconsolable crying lasting ≥3 hours within 48 hours after a previous pertussis-containing vaccine. (5.2) -seizures within 3 days after a previous pertussis-containing vaccine. (5.2) -If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following DAPTACEL. (5.3) For infants and children with a history of previous seizures, an antipyretic may be administered (in the dosage recommended in its prescribing information) at the time of vaccination with DAPTACEL and for the next 24 hours. (5.4) Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including DAPTACEL, to an infant born prematurely should be based on consideration of the individual infant's medical status and the potential benefits and possible risks of vaccination. (5.7) Syncope (fainting) has been reported following vaccination with DAPTACEL. Procedures should be in place to prevent falling injury and manage syncopal reactions. Side Effects Rates of adverse reactions varied by dose number, with systemic reactions most frequent following doses 1-3 and injection site reactions most frequent following doses 4 and 5. Systemic reactions that occurred in >50% of subjects following any dose included fussiness/irritability, inconsolable crying, and decreased activity/lethargy. Fever ≥38.0°C occurred in 6-16% of US subjects, depending on dose number. Injection site reactions that occurred in >30% of subjects following any dose included tenderness, redness and increase in arm circumference. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc., at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 and http://vaers.hhs.gov. DRUG INTERACTIONS Do not mix with any other vaccine in the same syringe or vial. (7.1) Immunosuppressive therapies may reduce the immune response to DAPTACEL. (7.2 )
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 DAPTACEL INDICATIONS AND USAGE
- 2 DAPTACEL DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 DAPTACEL CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Management of Acute Allergic Reactions
- 5.2 Adverse Reactions Following Prior Pertussis Vaccination
- 5.3 Guillain-Barré Syndrome and Brachial Neuritis
- 5.4 Infants and Children with a History of Previous Seizures
- 5.5 Limitations of Vaccine Effectiveness
- 5.6 Altered Immunocompetence
- 5.7 Apnea in Premature Infants
- 5.8 Syncope
- 6 DAPTACEL ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 11 DAPTACEL DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NON-CLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
DAPTACEL® is a vaccine indicated for active immunization against diphtheria, tetanus and pertussis as a five-dose series in infants and children 6 weeks through 6 years of age (prior to seventh birthday).
2 DOSAGE AND ADMINISTRATION
2.1 Immunization Series
DAPTACEL vaccine is to be administered as a 5 dose series at 2, 4 and 6 months of age (at intervals of 6-8 weeks), at 15-20 months of age and at 4-6 years of age. The first dose may be given as early as 6 weeks of age. Four doses of DAPTACEL vaccine constitute a primary immunization course for pertussis. The fifth dose is a booster for pertussis immunization. Three doses of DAPTACEL vaccine constitute a primary immunization course for diphtheria and tetanus. The fourth and fifth doses are boosters for diphtheria and tetanus immunization. [See Clinical Studies (14.1, 14.2, 14.3) .]
DAPTACEL vaccine should be used as the fifth dose of the DTaP series in children who initially received 4 doses of Pentacel® [(Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus and Haemophilus b Conjugate (Tetanus Toxoid Conjugate) vaccine, Sanofi Pasteur Limited]. Pentacel and DAPTACEL vaccines contain the same pertussis antigens, manufactured by the same process, although Pentacel vaccine contains twice the amount of detoxified pertussis toxin (PT) and four times the amount of filamentous hemagglutinin (FHA) as DAPTACEL vaccine.
Data are not available on the safety and effectiveness of using mixed sequences of DAPTACEL vaccine and DTaP vaccines from different manufacturers for successive doses of the DTaP vaccination series. DAPTACEL vaccine may be used to complete the immunization series in infants who have received 1 or more doses of whole-cell pertussis DTP. However, the safety and efficacy of DAPTACEL vaccine in such infants have not been fully demonstrated.
If a decision is made to withhold any recommended dose of pertussis vaccine, [see Contraindications (4.2), (4.3) and Warnings and Precautions (5.2) ], Diphtheria and Tetanus Toxoids Adsorbed For Pediatric Use (DT) should be administered.
2.2 Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, the product should not be administered.
After removing the "flip-off" cap, cleanse the vaccine vial stopper with a suitable germicide. Do not remove either the rubber stopper or the metal seal holding it in place. Just before use, shake the vial well, until a uniform, white, cloudy suspension results.
Using a sterile needle and syringe and aseptic technique, withdraw and administer a single 0.5 mL dose of DAPTACEL vaccine intramuscularly. Use a separate sterile needle and syringe for each injection. Changing needles between withdrawing the vaccine from the vial and injecting it into a recipient is not necessary unless the needle has been damaged or contaminated. In infants younger than 1 year, the anterolateral aspect of the thigh provides the largest muscle and is the preferred site of injection. In older children, the deltoid muscle is usually large enough for injection. The vaccine should not be injected into the gluteal area or areas where there may be a major nerve trunk.
Do not administer this product intravenously or subcutaneously.
DAPTACEL vaccine should not be combined through reconstitution or mixed with any other vaccine.
3 DOSAGE FORMS AND STRENGTHS
DAPTACEL vaccine is a suspension for injection in 0.5 mL single dose vials. See Description (11) for a complete listing of ingredients.
4 CONTRAINDICATIONS
4.1 Hypersensitivity
A severe allergic reaction (eg, anaphylaxis) after a previous dose of DAPTACEL vaccine or any other tetanus toxoid, diphtheria toxoid, or pertussis-containing vaccine, or any other component of this vaccine is a contraindication to administration of DAPTACEL vaccine. [See Description (11).] Because of uncertainty as to which component of the vaccine may be responsible, none of the components should be administered. Alternatively, such individuals may be referred to an allergist for evaluation if further immunizations are to be considered.
4.2 Encephalopathy
Encephalopathy (eg, coma, decreased level of consciousness, prolonged seizures) within 7 days of a previous dose of a pertussis containing vaccine that is not attributable to another identifiable cause is a contraindication to administration of any pertussis-containing vaccine, including DAPTACEL vaccine.
4.3 Progressive Neurologic Disorder
Progressive neurologic disorder, including infantile spasms, uncontrolled epilepsy, or progressive encephalopathy is a contraindication to administration of any pertussis-containing vaccine, including DAPTACEL vaccine. Pertussis vaccine should not be administered to individuals with such conditions until a treatment regimen has been established and the condition has stabilized.
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Epinephrine hydrochloride solution (1:1,000) and other appropriate agents and equipment must be available for immediate use in case an anaphylactic or acute hypersensitivity reaction occurs.
5.2 Side Effects Following Prior Pertussis Vaccination
If any of the following events occur within the specified period after administration of a whole-cell pertussis vaccine or a vaccine containing an acellular pertussis component, the decision to administer DAPTACEL vaccine should be based on careful consideration of potential benefits and possible risks. [See Dosage and Administration (2.1).]
- Temperature of ≥40.5°C (105°F) within 48 hours, not attributable to another identifiable cause.
- Collapse or shock-like state (hypotonic-hyporesponsive episode (HHE)) within 48 hours.
- Persistent, inconsolable crying lasting ≥3 hours within 48 hours.
- Seizures with or without fever within 3 days.
5.3 Guillain-Barré Syndrome and Brachial Neuritis
A review by the Institute of Medicine found evidence for a causal relation between tetanus toxoid and both brachial neuritis and Guillain-Barré syndrome. (1) If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following DAPTACEL vaccine.
5.4 Infants and Children with a History of Previous Seizures
For infants or children with a history of previous seizures, an appropriate antipyretic may be administered (in the dosage recommended in its prescribing information) at the time of vaccination with a vaccine containing an acellular pertussis component (including DAPTACEL vaccine) and for the following 24 hours, to reduce the possibility of post-vaccination fever.
5.5 Limitations of Vaccine Effectiveness
Vaccination with DAPTACEL vaccine may not protect all individuals.
5.6 Altered Immunocompetence
If DAPTACEL vaccine is administered to immunocompromised persons, including persons receiving immunosuppressive therapy, the expected immune response may not be obtained. [See Immunosuppressive Treatments (7.2).]
5.7 Apnea in Premature Infants
Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including DAPTACEL, to an infant born prematurely should be based on consideration of the individual infant's medical status and the potential benefits and possible risks of vaccination.
5.8 Syncope
Syncope (fainting) has been reported following vaccination with DAPTACEL. Procedures should be in place to prevent falling injury and manage syncopal reactions.
6 ADVERSE REACTIONS
6.1 Data from Clinical Studies
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to vaccine use and for approximating rates of those events.
Approximately 18,000 doses of DAPTACEL vaccine have been administered to infants and children in 9 clinical studies. Of these, 3 doses of DAPTACEL vaccine were administered to 4,998 children, 4 doses of DAPTACEL vaccine were administered to 1,725 children, and 5 doses of DAPTACEL vaccine were administered to 485 children. A total of 989 children received 1 dose of DAPTACEL vaccine following 4 prior doses of Pentacel vaccine.
In a randomized, double-blinded pertussis vaccine efficacy trial, the Sweden I Efficacy Trial, conducted in Sweden during 1992-1995, the safety of DAPTACEL vaccine was compared with DT and a whole-cell pertussis DTP vaccine. A standard diary card was kept for 14 days after each dose and follow-up telephone calls were made 1 and 14 days after each injection. Telephone calls were made monthly to monitor the occurrence of severe events and/or hospitalizations for the 2 months after the last injection. There were fewer of the solicited common local and systemic reactions following DAPTACEL vaccine than following the whole-cell pertussis DTP vaccine. As shown in Table 1, the 2,587 infants who received DAPTACEL vaccine at 2, 4 and 6 months of age had similar rates of reactions within 24 hours as recipients of DT and significantly lower rates than infants receiving whole-cell pertussis DTP.
| Dose 1 (2 MONTHS) |
Dose 2 (4 MONTHS) |
Dose 3 (6 MONTHS) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| EVENT | DAPTACEL vaccine N = 2,587 |
DT N = 2,574 |
DTP N = 2,102 |
DAPTACEL vaccine N = 2,563 |
DT N = 2,555 |
DTP N = 2,040 |
DAPTACEL vaccine N = 2,549 |
DT N = 2,538 |
DTP N = 2,001 |
| DT: Swedish National Biologics Laboratories DTP: whole-cell pertussis DTP, Sanofi Pasteur Inc. N = Number of evaluable subjects |
|||||||||
| Local | |||||||||
| Tenderness (Any) |
8.0 |
8.4 | 59.5 | 10.1 |
10.3 | 60.2 | 10.8 |
10.0 | 50.0 |
| Redness ≥2 cm |
0.3 |
0.3 | 6.0 | 1.0 |
0.8 | 5.1 | 3.7 |
2.4 | 6.4 |
| Swelling ≥2 cm |
0.9 |
0.7 | 10.6 | 1.6 |
2.0 | 10.0 | 6.3 |
3.9 | 10.5 |
| Systemic | |||||||||
| Fever ≥38°C (100.4°F) |
7.8 |
7.6 | 72.3 | 19.1 |
18.4 | 74.3 | 23.6 |
22.1 | 65.1 |
| Fretfulness |
32.3 | 33.0 | 82.1 | 39.6 | 39.8 | 85.4 | 35.9 | 37.7 | 73.0 |
| Anorexia | 11.2 |
10.3 | 39.2 | 9.1 |
8.1 | 25.6 | 8.4 |
7.7 | 17.5 |
| Drowsiness | 32.7 |
32.0 | 56.9 | 25.9 |
25.6 | 50.6 | 18.9 |
20.6 | 37.6 |
| Crying ≥1 hour |
1.7 |
1.6 | 11.8 | 2.5 |
2.7 | 9.3 | 1.2 |
1.0 | 3.3 |
| Vomiting | 6.9 |
6.3 | 9.5 | 5.2 |
5.8 | 7.4 | 4.3 | 5.2 | 5.5 |
The incidence of serious and less common selected systemic events in the Sweden I Efficacy Trial is summarized in Table 2.
| EVENT | Dose 1 (2 MONTHS) |
Dose 2 (4 MONTHS) |
Dose 3 (6 MONTHS) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| DAPTACEL vaccine N = 2,587 |
DT N = 2,574 |
DTP N = 2,102 |
DAPTACEL vaccine N = 2,565 |
DT N = 2,556 |
DTP N = 2,040 |
DAPTACEL vaccine N = 2,551 |
DT N = 2,539 |
DTP N = 2,002 |
|
| DT: Swedish National Biologics Laboratories DTP: whole-cell pertussis DTP, Sanofi Pasteur Inc. N = Number of evaluable subjects |
|||||||||
| Rectal temperature ≥40°C (104°F) within 48 hours of vaccination | 0.39 | 0.78 | 3.33 | 0 | 0.78 | 3.43 | 0.39 | 1.18 | 6.99 |
| Hypotonic-hypo-responsive episode within 24 hours of vaccination | 0 | 0 | 1.9 | 0 | 0 | 0.49 | 0.39 | 0 | 0 |
| Persistent crying ≥3 hours within 24 hours of vaccination | 1.16 | 0 | 8.09 | 0.39 | 0.39 | 1.96 | 0 | 0 | 1.0 |
| Seizures within 72 hours of vaccination | 0 | 0.39 | 0 | 0 | 0.39 | 0.49 | 0 | 0.39 | 0 |
In the Sweden I Efficacy Trial, one case of whole limb swelling and generalized symptoms, with resolution within 24 hours, was observed following dose 2 of DAPTACEL vaccine. No episodes of anaphylaxis or encephalopathy were observed. No seizures were reported within 3 days of vaccination with DAPTACEL vaccine. Over the entire study period, 6 seizures were reported in the DAPTACEL vaccine group, 9 in the DT group and 3 in the whole-cell pertussis DTP group, for overall rates of 2.3, 3.5 and 1.4 per 1,000 vaccinees, respectively. One case of infantile spasms was reported in the DAPTACEL vaccine group. There were no instances of invasive bacterial infection or death.
In a US study, children received 4 doses of DAPTACEL vaccine at 2, 4, 6 and 15-17 months of age. A total of 1,454 children received DAPTACEL vaccine and were included in the safety analyses. Of these, 51.7% were female, 77.2% Caucasian, 6.3% Black, 6.5% Hispanic, 0.9% Asian and 9.1% other races. The use of DAPTACEL vaccine as a fifth dose of DTaP vaccine was evaluated in 2 subsequent US clinical studies. In one study, a total of 485 children received DAPTACEL vaccine at 4-6 years of age following 4 prior doses of DAPTACEL vaccine in infancy (DAPTACEL-primed). In a separate study, a total of 989 children received DAPTACEL vaccine at 4-6 years of age following 4 prior doses of Pentacel vaccine in infancy (Pentacel-primed). The children included in these fifth dose studies were non-random subsets of participants from previous DAPTACEL or Pentacel studies. The subsets were representative of all children who received 4 doses of DAPTACEL or Pentacel vaccine in the earlier studies with regard to frequencies of solicited local and systemic adverse events following the fourth dose.
In the US 4-dose DAPTACEL study, at 2, 4, and 6 months of age, DAPTACEL vaccine was administered concomitantly with Haemophilus influenzae type b (Hib) conjugate vaccine (tetanus toxoid conjugate) (Sanofi Pasteur SA), inactivated poliovirus vaccine (IPV) (Sanofi Pasteur SA), and 7-valent pneumococcal conjugate vaccine (Wyeth Pharmaceuticals Inc.). Infants had received the first dose of hepatitis B vaccine at 0 months of age. At 2 and 6 months of age, hepatitis B vaccine (recombinant) (Merck & Co., Inc.) was also administered concomitantly with DAPTACEL vaccine. Based on random assignment, the fourth dose of DAPTACEL vaccine was administered either alone; concomitantly with Hib conjugate (tetanus toxoid conjugate) vaccine; or concomitantly with Hib conjugate (tetanus toxoid conjugate) vaccine, 7-valent pneumococcal conjugate vaccine, measles, mumps, rubella (MMR) vaccine (Merck & Co., Inc.), and varicella vaccine (Merck & Co., Inc.). In the fifth dose studies, DAPTACEL vaccine was administered concomitantly with IPV (all DAPTACEL-primed subjects and 47% of Pentacel-primed subjects) and MMR vaccine.
In the US studies, the occurrence of solicited local and systemic adverse events listed in Table 3 was recorded daily by parents or guardians for Days 0-7 following vaccination. For Days 0 and 1 following the first three doses of DAPTACEL vaccine, signs and symptoms of HHE also were solicited. Periodic telephone calls were made to inquire about adverse events. Serious adverse events were monitored during the three studies, through 6 months following the last dose of DAPTACEL vaccine.
The incidence and severity of selected solicited local and systemic adverse events that occurred within 3 days following each dose of DAPTACEL vaccine are shown in Table 3. The incidence of redness, tenderness and swelling at the DAPTACEL injection site increased with the fourth and fifth doses, with the highest rates reported after the fifth dose. The incidence of redness, tenderness and swelling at the DAPTACEL injection site was similarly increased when DAPTACEL vaccine was given as a fifth dose of DTaP vaccine in Pentacel-primed children.
Dose 1 |
Dose 2 |
Dose 3 |
Dose 4 |
Dose 5 | ||
|---|---|---|---|---|---|---|
DAPTACEL-primed |
Pentacel-primed |
|||||
| N = 1390-1406 % |
N = 1346-1360 % |
N = 1301-1312 % |
N = 1118-1144 % |
N = 473-481 % |
N = 936-981 % |
|
| Injection Site Reactions (DAPTACEL vaccine injection site) | ||||||
| Redness | ||||||
| >5 mm | 6.2 | 7.1 | 9.6 | 17.3 | 35.8 | 20.2 |
| 25 - 50 mm | 0.6 | 0.5 | 1.9 | 6.3 | 10.4 | 6.8 |
| >50 mm | 0.4 | 0.1 | 0.0 | 3.1 | 15.8 | 6.6 |
| Swelling | ||||||
| >5 mm | 4.0 | 4.0 | 6.5 | 11.7 | 23.9 | 12.0 |
| 25 - 50 mm | 1.2 | 0.6 | 1.0 | 3.2 | 5.8 | 4.1 |
| >50 mm | 0.4 | 0.1 | 0.1 | 1.6 | 7.7 | 2.9 |
|
Tenderness
Dose 5 - Moderate: interfered with activities, but did not require medical care or absenteeism; Severe: incapacitating, unable to perform usual activities, may have/or required medical care or absenteeism. |
||||||
| Any | 48.8 | 38.2 | 40.9 | 49.5 | 61.5 | 50.0 |
| Moderate | 16.5 | 9.9 | 10.6 | 12.3 | 11.2 | 7.4 |
| Severe | 4.1 | 2.3 | 1.7 | 2.2 | 1.7 | 0.3 |
|
Increase in Arm Circumference
|
||||||
| >5 mm | - | - | - | 30.1 | 38.3 | 28.6 |
| 20 - 40 mm | 7.0 | 14.0 | 7.6 | |||
| >40 mm | 0.4 | 1.5 | 1.2 | |||
|
Interference with Normal Activity of the Arm
|
||||||
| Any | - | - | - | - | 20.4 | 8.8 |
| Moderate | 5.6 | 1.7 | ||||
| Severe | 0.4 | 0.0 | ||||
| Systemic Reactions | ||||||
|
Fever
|
||||||
| ≥38.0°C | 9.3 | 16.1 | 15.8 | 10.5 | 6.1 | 4.6 |
| >38.5-39.5°C | 1.5 | 3.9 | 4.8 | 2.7 | 2.1 | 2.0 |
| >39.5°C | 0.1 | 0.4 | 0.3 | 0.7 | 0.2 | 0.2 |
|
Decreased Activity/Lethargy
Dose 5 - Moderate: interfered with activities, but did not require medical care or absenteeism; Severe: incapacitating, unable to perform usual activities, may have/or required medical care or absenteeism. |
||||||
| Any | 51.1 | 37.4 | 33.2 | 25.3 | 21.0 | 12.6 |
| Moderate | 23.0 | 14.4 | 12.1 | 8.2 | 5.8 | 3.6 |
| Severe | 1.2 | 1.4 | 0.6 | 1.0 | 0.8 | 0.4 |
|
Inconsolable Crying
Dose 5 - Moderate: interfered with activities, but did not require medical care or absenteeism; Severe: incapacitating, unable to perform usual activities, may have/or required medical care or absenteeism. |
||||||
| Any | 58.5 | 51.4 | 47.9 | 37.1 | 14.1 | 7.2 |
| Moderate | 14.2 | 12.6 | 10.8 | 7.7 | 3.5 | 1.9 |
| Severe | 2.2 | 3.4 | 1.4 | 1.5 | 0.4 | 0.3 |
|
Fussiness/Irritability
Dose 5 - Moderate: interfered with activities, but did not require medical care or absenteeism; Severe: incapacitating, unable to perform usual activities, may have/or required medical care or absenteeism. |
||||||
| Any | 75.8 | 70.7 | 67.1 | 54.4 | 34.9 | 22.9 |
| Moderate | 27.7 | 25.0 | 22.0 | 16.3 | 7.5 | 5.3 |
| Severe | 5.6 | 5.5 | 4.3 | 3.9 | 0.4 | 0.5 |
In the US study in which children received 4 doses of DAPTACEL vaccine, of 1,454 subjects who received DAPTACEL vaccine, 5 (0.3%) subjects experienced a seizure within 60 days following any dose of DAPTACEL vaccine. One seizure occurred within 7 days post-vaccination: an infant who experienced an afebrile seizure with apnea on the day of the first vaccination. Three other cases of seizures occurred between 8 and 30 days post-vaccination. Of the seizures that occurred within 60 days post-vaccination, 3 were associated with fever. In this study, there were no reported cases of HHE following DAPTACEL vaccine. There was one death due to aspiration 222 days post-vaccination in a subject with ependymoma. Within 30 days following any dose of DAPTACEL vaccine, 57 (3.9%) subjects reported at least one serious adverse event. During this period, the most frequently reported serious adverse event was bronchiolitis, reported in 28 (1.9%) subjects. Other serious adverse events that occurred within 30 days following DAPTACEL vaccine include three cases of pneumonia, two cases of meningitis and one case each of sepsis, pertussis (post-dose 1), irritability and unresponsiveness.
In the US study in which DAPTACEL vaccine was administered as a fifth DTaP dose in DAPTACEL-primed subjects, within 30 days following the fifth consecutive dose of DAPTACEL vaccine, 1 (0.2%) subject reported 2 serious adverse events (bronchospasm and hypoxia). In the US study in which DAPTACEL vaccine was administered as a fifth DTaP dose in Pentacel-primed subjects, within 30 days following DAPTACEL, 4 (0.4%) subjects reported one or more serious adverse events (asthma and pneumonia; idiopathic thrombocytopenic purpura; vomiting; cellulitis not at the injection site). In these two studies, there were no reports of seizures within 30 days following DAPTACEL vaccine in either the DAPTACEL-primed subjects or Pentacel-primed subjects.
In another study (Sweden II Efficacy Trial), 3 DTaP vaccines and a whole-cell pertussis DTP vaccine, none of which are licensed in the US, were evaluated to assess relative safety and efficacy. This study included HCPDT, a vaccine made of the same components as DAPTACEL vaccine but containing twice the amount of detoxified PT and four times the amount of FHA (20 mcg detoxified PT and 20 mcg FHA). HHE was observed following 29 (0.047%) of 61,220 doses of HCPDT; 16 (0.026%) of 61,219 doses of an acellular pertussis vaccine made by another manufacturer; and 34 (0.056%) of 60,792 doses of a whole-cell pertussis DTP vaccine. There were 4 additional cases of HHE in other studies using HCPDT vaccine for an overall rate of 33 (0.047%) in 69,525 doses.
6.2 Data from Post-Marketing Experience
The following adverse events have been spontaneously reported during the post-marketing use of DAPTACEL vaccine in the US and other countries. Because these events are reported voluntarily from a population of uncertain size, it may not be possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
The following adverse events were included based on one or more of the following factors: severity, frequency of reporting, or strength of evidence for a causal relationship to DAPTACEL vaccine.
-
Blood and lymphatic disorders
Lymphadenopathy -
Cardiac disorders
Cyanosis -
Gastro-intestinal disorders
Nausea, diarrhea -
General disorders and administration site conditions
Local reactions: injection site pain, injection site rash, injection site nodule, injection site mass, extensive swelling of injected limb (including swelling that involves adjacent joints). -
Infections and infestations
Injection site cellulitis, cellulitis, injection site abscess -
Immune system disorders
Hypersensitivity, allergic reaction, anaphylactic reaction (edema, face edema, swelling face, pruritus, rash generalized) and other types of rash (erythematous, macular, maculo-papular) -
Nervous system disorders
Convulsions: febrile convulsion, grand mal convulsion, partial seizures
HHE, hypotonia, somnolence, syncope -
Psychiatric disorders
Screaming
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Other Vaccines
In clinical trials, DAPTACEL vaccine was administered concomitantly with one or more of the following US licensed vaccines: Hib conjugate vaccine, IPV, hepatitis B vaccine, pneumococcal conjugate vaccine, MMR vaccine, and varicella vaccine. [See Adverse Reactions (6.1) and Clinical Studies (14).] When DAPTACEL vaccine is given at the same time as another injectable vaccine(s), the vaccines should be administered with different syringes and at different injection sites.
7.2 Immunosuppressive Treatments
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids (used in greater than physiologic doses), may reduce the immune response to DAPTACEL vaccine.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with DAPTACEL vaccine. It is also not known whether DAPTACEL vaccine can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity.
8.4 Pediatric Use
DAPTACEL vaccine is not indicated for infants below 6 weeks of age or children 7 years of age or older. Safety and effectiveness of DAPTACEL vaccine in these age groups have not been established.
11 DESCRIPTION
DAPTACEL vaccine is a sterile isotonic suspension of pertussis antigens and diphtheria and tetanus toxoids adsorbed on aluminum phosphate, for intramuscular injection.
Each 0.5 mL dose contains 15 Lf diphtheria toxoid, 5 Lf tetanus toxoid and acellular pertussis antigens [10 mcg detoxified pertussis toxin (PT), 5 mcg filamentous hemagglutinin (FHA), 3 mcg pertactin (PRN), and 5 mcg fimbriae types 2 and 3 (FIM)].
Other ingredients per 0.5 mL dose include 1.5 mg aluminum phosphate (0.33 mg of aluminum) as the adjuvant, ≤5 mcg residual formaldehyde, <50 ng residual glutaraldehyde and 3.3 mg (0.6% v/v) 2-phenoxyethanol (not as a preservative).
The acellular pertussis vaccine components are produced from Bordetella pertussis cultures grown in Stainer-Scholte medium (2) modified by the addition of casamino acids and dimethyl-beta-cyclodextrin. PT, FHA and PRN are isolated separately from the supernatant culture medium. The FIM components are extracted and co-purified from the bacterial cells. The pertussis antigens are purified by sequential filtration, salt-precipitation, ultrafiltration and chromatography. PT is detoxified with glutaraldehyde. FHA is treated with formaldehyde, and the residual aldehydes are removed by ultrafiltration. The individual antigens are adsorbed separately onto aluminum phosphate.
Corynebacterium diphtheriae is grown in modified Mueller's growth medium. (3) After purification by ammonium sulfate fractionation, diphtheria toxin is detoxified with formaldehyde and diafiltered. Clostridium tetani is grown in modified Mueller-Miller casamino acid medium without beef heart infusion. (4) Tetanus toxin is detoxified with formaldehyde and purified by ammonium sulfate fractionation and diafiltration. Diphtheria and tetanus toxoids are individually adsorbed onto aluminum phosphate.
The adsorbed diphtheria, tetanus and acellular pertussis components are combined with aluminum phosphate (as adjuvant), 2-phenoxyethanol (not as a preservative) and water for injection.
Both diphtheria and tetanus toxoids induce at least 2 units of antitoxin per mL in the guinea pig potency test. The potency of the acellular pertussis vaccine components is determined by the antibody response of immunized mice to detoxified PT, FHA, PRN and FIM as measured by enzyme-linked immunosorbent assay (ELISA).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Diphtheria
Diphtheria is an acute toxin-mediated disease caused by toxigenic strains of C diphtheriae. Protection against disease is due to the development of neutralizing antibodies to diphtheria toxin. A serum diphtheria antitoxin level of 0.01 IU/mL is the lowest level giving some degree of protection. Antitoxin levels of at least 0.1 IU/mL are generally regarded as protective. (5) Levels of 1.0 IU/mL have been associated with long-term protection. (6)
Tetanus
Tetanus is an acute disease caused by an extremely potent neurotoxin produced by C tetani. Protection against disease is due to the development of neutralizing antibodies to tetanus toxin. A serum tetanus antitoxin level of at least 0.01 IU/mL, measured by neutralization assay is considered the minimum protective level. (5) (7) A tetanus antitoxin level ≥0.1 IU/mL as measured by the ELISA used in clinical studies of DAPTACEL vaccine is considered protective.
Pertussis
Pertussis (whooping cough) is a respiratory disease caused by B pertussis. This Gram-negative coccobacillus produces a variety of biologically active components, though their role in either the pathogenesis of, or immunity to, pertussis has not been clearly defined.
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
DAPTACEL vaccine has not been evaluated for carcinogenic or mutagenic potential or impairment of fertility.
14 CLINICAL STUDIES
14.1 Diphtheria
In a US study in which children received 4 doses of DAPTACEL vaccine at 2, 4, 6 and 15-17 months of age, after the third dose, 100% (N = 1,099) achieved diphtheria antitoxin levels of ≥0.01 IU/mL and 98.5% achieved diphtheria antitoxin levels of ≥0.10 IU/mL. Among a random subset of children who received the fourth dose of DAPTACEL vaccine at 15-16 months of age, 96.5% (N = 659) achieved diphtheria antitoxin levels of ≥1.0 IU/mL after the fourth dose.
14.2 Tetanus
In a US study in which children received 4 doses of DAPTACEL vaccine at 2, 4, 6 and 15-17 months of age, after the third dose, 100% (N = 1,037) achieved tetanus antitoxin levels of ≥0.10 IU/mL. Among a random subset of children who received the fourth dose of DAPTACEL vaccine at 15-16 months of age, 98.8% (N = 681) achieved tetanus antitoxin levels of ≥1.0 IU/mL after the fourth dose.
14.3 Pertussis
A randomized, double-blinded, placebo-controlled efficacy and safety study was conducted in Sweden during 1992-1995 (Sweden I Efficacy Trial) under the sponsorship of the National Institute of Allergy and Infectious Diseases. A total of 9,829 infants received 1 of 4 vaccines: DAPTACEL vaccine (N = 2,587); another investigational acellular pertussis vaccine (N = 2,566); whole-cell pertussis DTP vaccine (N = 2,102); or DT vaccine as placebo (Swedish National Bacteriological Laboratory, N = 2,574). Infants were immunized at 2, 4 and 6 months of age. The mean length of follow-up was 2 years after the third dose of vaccine. The protective efficacy of DAPTACEL vaccine against pertussis after 3 doses using the World Health Organization (WHO) case definition (≥21 consecutive days of paroxysmal cough with culture or serologic confirmation or epidemiologic link to a confirmed case) was 84.9% (95% confidence interval [CI] 80.1 to 88.6). The protective efficacy of DAPTACEL vaccine against mild pertussis (≥1 day of cough with laboratory confirmation) was 77.9% (95% CI 72.6 to 82.2). Protection against pertussis by DAPTACEL vaccine was sustained for the 2-year follow-up period.
In order to assess the antibody response to the pertussis antigens of DAPTACEL vaccine in the US population, 2 lots of DAPTACEL vaccine, including the lot used in the Sweden I Efficacy Trial, were administered to US infants in the US Bridging Study. In this study, antibody responses following 3 doses of DAPTACEL vaccine given to US children at 2, 4 and 6 months of age were compared to those from a subset of the infants enrolled in the Sweden I Efficacy Trial. Assays were performed in parallel on the available sera from the US and Swedish infants. Antibody responses to all the antigens were similar except for those to the PRN component. For both lots of DAPTACEL vaccine, the geometric mean concentration (GMC) and percent response to PRN in US infants (Lot 006, N = 107; Lot 009, N = 108) were significantly lower after 3 doses of vaccine than in Swedish infants (N = 83). In separate US and Canadian studies in which children received DAPTACEL vaccine at 2, 4 and 6 months of age, with a fourth dose at either 17-20 months (Canadian study) or 15-16 months (random subset from US study) of age, antibody responses to each pertussis antigen following the fourth dose (Canadian study N = 275; US study N = 237-347) were at least as high as those seen in the Swedish infants after 3 doses. While a serologic correlate of protection for pertussis has not been established, the antibody response to all antigens in North American infants after 4 doses of DAPTACEL vaccine at 2, 4, 6 and 15-20 months of age was comparable to that achieved in Swedish infants in whom efficacy was demonstrated after 3 doses of DAPTACEL vaccine at 2, 4 and 6 months of age.
14.4 Concomitantly Administered Vaccines
In the US Bridging study, DAPTACEL vaccine was given concomitantly with Hib conjugate vaccine (Sanofi Pasteur SA) according to local practices. Anti-PRP immune response was evaluated in 261 infants who received 3 doses of Hib conjugate vaccine. One month after the third dose, 96.9% achieved anti-PRP antibody levels of at least 0.15 mcg/mL and 82.7% achieved antibody levels of at least 1.0 mcg/mL.
In the US study in which infants received DAPTACEL vaccine concomitantly with Hib conjugate (tetanus toxoid conjugate) vaccine, IPV, 7-valent pneumococcal conjugate vaccine, and hepatitis B vaccine [see Adverse Reactions (6.1) ], at 7 months of age, 100.0% of subjects (N = 1,050-1,097) had protective neutralizing antibody levels (≥1:8 1/dil) for poliovirus types 1, 2 and 3; and 92.4% (N = 998) achieved anti-hepatitis B surface antigen levels ≥10.0 mIU/mL. Although there is no established serologic correlate of protection for any of the pneumococcal serotypes, at 7 months of age 91.3%-98.9% (N = 1,027-1,029) achieved anti-pneumococcal polysaccharide levels ≥0.5 mcg/mL for serotypes 4, 9V, 14, 18C, 19F and 23F and 80.7% (N = 1,027) achieved an anti-pneumococcal polysaccharide level ≥0.5 mcg/mL for serotype 6B. The mumps seroresponse rate was lower when DAPTACEL vaccine was administered concomitantly (86.6%; N = 307) vs. non-concomitantly (90.1%; N = 312) with the first dose of MMR vaccine [upper limit of 90% confidence interval for difference in rates (non-concomitant minus concomitant) >5%]. There was no evidence for interference in the immune response to the measles, rubella, and varicella antigens or to the fourth dose of the 7-valent pneumococcal conjugate vaccine with concomitant administration of DAPTACEL vaccine.
15 REFERENCES
-
1 Stratton KR, et al. editors. Adverse events associated with childhood vaccines; evidence bearing on causality. Washington D.C.: National Academy Press. 1994. p. 67-117. -
2 Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol 1970;63:211-20. -
3 Stainer DW. Production of diphtheria toxin. In: Manclark CR, editor. Proceedings of an informal consultation on the World Health Organization requirements for diphtheria, tetanus, pertussis and combined vaccines. United States Public Health Service, Bethesda, MD. DHHS 91-1174. 1991. p. 7-11. -
4 Mueller JH, Miller PA. Variable factors influencing the production of tetanus toxin. J Bacteriol 1954;67(3):271-7. -
5 Department of Health and Human Services, Food and Drug Administration. Biological products; bacterial vaccines and toxoids; implementation of efficacy review; proposed rule. Federal Register 1985;50(240):51002-117. -
6 Wharton M, et al. Diphtheria Toxoid. In: Plotkin SA, Orenstein WA, editors. Vaccines. 4th ed. Philadelphia, PA: W. B. Saunders 2004 p. 211-28. -
7 Wassilak SGF, et al. Tetanus Toxoid. In: Plotkin SA, Orenstein WA, editors. Vaccines. 4th ed. Philadelphia, PA: W. B. Saunders 2004 p. 745-81.
16 HOW SUPPLIED/STORAGE AND HANDLING
The vial stopper for this product is not made with natural rubber latex.
DAPTACEL vaccine is supplied in a single dose vial (NDC No. 49281-286-58):
in packages of 1 vial: NDC No. 49281-286-01;
in packages of 5 vials: NDC No. 49281-286-05;
in packages of 10 vials: NDC No. 49281-286-10.
DAPTACEL vaccine should be stored at 2° to 8°C (35° to 46°F). DO NOT FREEZE. Product which has been exposed to freezing should not be used. Do not use after expiration date shown on the label.
17 PATIENT COUNSELING INFORMATION
Before administration of DAPTACEL vaccine, health-care personnel should inform the parent or guardian of the benefits and risks of the vaccine and the importance of completing the immunization series unless a contraindication to further immunization exists.
The health-care provider should inform the parent or guardian about the potential for adverse reactions that have been temporally associated with DAPTACEL vaccine and other vaccines containing similar components. The health-care provider should provide the Vaccine Information Statements (VIS) which are required by the National Childhood Vaccine Injury Act of 1986 to be given with each immunization. The parent or guardian should be instructed to report adverse reactions to their health-care provider.
Manufactured by:
Sanofi Pasteur Limited
Toronto Ontario Canada
Distributed by:
Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
US Patents: 4500639, 4687738, 4784589, 4997915, 5444159, 5667787, 5877298.
DAPTACEL® is a registered trademark of the sanofi pasteur group, and its subsidiaries.
R9-1013 USA
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Label
6 wks - 6 yrs
DTaP
NDC 49281-286-58
Diphtheria and Tetanus
Toxoids and Acellular
Pertussis Vaccine
Adsorbed
DAPTACEL®
Rx only
IM only
1 Dose (0.5 mL)
Sanofi Pasteur Limited

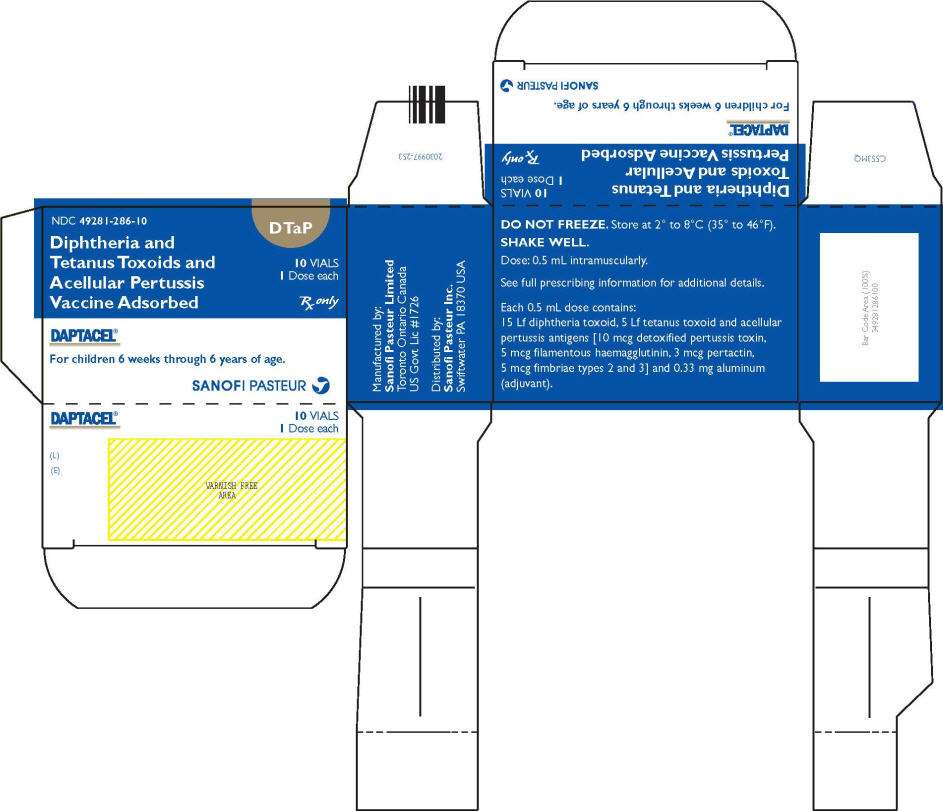
PRINCIPAL DISPLAY PANEL - 10 Vial Carton
NDC 49281-286-10
Diphtheria and
Tetanus Toxoids and
Acellular Pertussis
Vaccine Adsorbed
DTaP
10 VIALS
1 Dose each
Rx only
DAPTACEL ®
For children 6 weeks through 6 years of age.
SANOFI PASTEUR
DAPTACELcorynebacterium diphtheriae toxoid antigen (formaldehyde inactivated), clostridium tetani toxoid antigen (formaldehyde inactivated), bordetella pertussis toxoid antigen (glutaraldehyde inactivated), bordetella pertussis filamentous hemagglutinin antigen (formaldehyde inactivated), bordetella pertussis pertactin antigen, and bordetella pertussis fimbriae 2/3 antigen INJECTION, SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||